Effect of Early Attainment of Everolimus Target Trough Levels on Efficacy-Safety Outcomes in De Novo Kidney Transplant Recipients: 12-Month Results from US92 and TRANSFORM Studies
1US92 Study Group, Salt Lake City
2TRANSFORM Study Group, East Hanover
3Novartis Pharmaceuticals Corporation, East Hanover.
Meeting: 2018 American Transplant Congress
Abstract number: 36
Keywords: Efficacy, Immunosuppression, Kidney transplantation, Safety
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: mTORi Based Regimens
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:06pm-3:18pm
Presentation Time: 3:06pm-3:18pm
Location: Room 6A
Purpose: Attainment of everolimus (EVR) target trough level (C0 3-8ng/mL) is crucial in achieving comparable efficacy-safety with EVR+reduced (r) calcineurin inhibitor regimen (CNI; tacrolimus [TAC] or cyclosporine A [CsA]) vs standard (s) CNI-based regimen in de novo kidney transplant recipients (KTR). Here, we evaluate the effect of 2 EVR dosing schedules on efficacy outcomes from 2 studies.
Methods: In US92 study, KTR were randomized <24h post-KT to EVR (starting dose 0.75mg bid; C0 3-8ng/mL)+rTAC or mycophenolic acid (MPA)+sTAC. In TRANSFORM study, KTR were randomized <24h post-KT to EVR+rCNI (EVR C0 3-8ng/mL: starting dose 1.5mg bid [TAC] or 0.75mg bid [CsA]) or MPA+sCNI (TAC/CsA). All patients in both the studies received basiliximab/antithymocyte globulin induction+steroids. Composite efficacy failure (CEF) of treated biopsy-proven acute rejection (tBPAR), graft loss or death, renal function (eGFR; MDRD4), and safety were evaluated at month (M)12 in US92 study and US cohort of the TRANSFORM study.
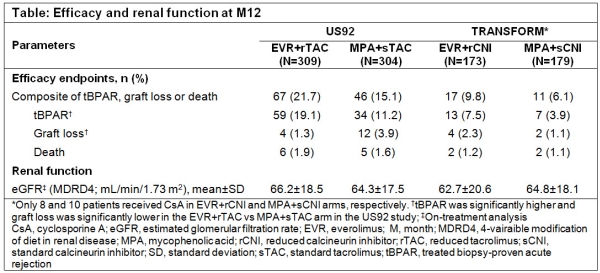
Results: Overall, 613 (EVR+rTAC: 309, MPA+sTAC: 304) and 352 (EVR+rCNI: 173 [CsA: 8], MPA+sCNI: 179 [CsA: 10]) KTR from US92 and TRANSFORM were considered for analysis, respectively. By Week 1, mean EVR C0 in the EVR+rTAC arm of US92 (3.2ng/mL) was comparatively lower than that of TRANSFORM (4.7ng/mL); thus, more patients in the EVR+rTAC arm of US92 (55.8%) than that of TRANSFORM (27.1%) were <3ng/mL target C0. Mean TAC C0 at M12 in the EVR+rTAC arm was near the upper limit of target C0 for both studies. M12 incidence of CEF in EVR arm was higher in US92 vs TRANSFORM (21.7% vs 9.8%; Table) and mainly driven by tBPAR (19.1% vs 7.5%). Mean eGFR and safety were comparable between studies.
Conclusion: EVR-facilitated CNI reduction was associated with comparable efficacy, safety and renal function as sCNI regimen. Moreover, higher EVR starting dose in TRANSFORM may have helped early target C0 attainment, thereby providing better anti-rejection efficacy.
CITATION INFORMATION: Shihab F., Qazi Y., Peddi V., Shaffer D., Kim D., McCague K., Patel D., Mulgaonkar S. Effect of Early Attainment of Everolimus Target Trough Levels on Efficacy-Safety Outcomes in De Novo Kidney Transplant Recipients: 12-Month Results from US92 and TRANSFORM Studies Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Shihab F, Qazi Y, Peddi V, Shaffer D, Kim D, McCague K, Patel D, Mulgaonkar S. Effect of Early Attainment of Everolimus Target Trough Levels on Efficacy-Safety Outcomes in De Novo Kidney Transplant Recipients: 12-Month Results from US92 and TRANSFORM Studies [abstract]. https://atcmeetingabstracts.com/abstract/effect-of-early-attainment-of-everolimus-target-trough-levels-on-efficacy-safety-outcomes-in-de-novo-kidney-transplant-recipients-12-month-results-from-us92-and-transform-studies/. Accessed March 6, 2026.« Back to 2018 American Transplant Congress