Early versus Delayed Cytomegalovirus Prophylaxis in High-Risk Kidney Transplant Recipients
A. Hutchins1, M. Leick2, M. Keck2, J. S. McMullen2
1Pharmacy, Houston Methodist Hospital, Houston, TX, 2Pharmacy, Nebraska Medicine, Omaha, NE
Meeting: 2022 American Transplant Congress
Abstract number: 1008
Keywords: Cytomeglovirus, Infection, Kidney transplantation, Prophylaxis
Topic: Clinical Science » Infection Disease » 25 - Kidney Infectious Non-Polyoma & Non-Viral Hepatitis
Session Information
Session Name: Kidney Infectious Non-Polyoma & Non-Viral Hepatitis
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
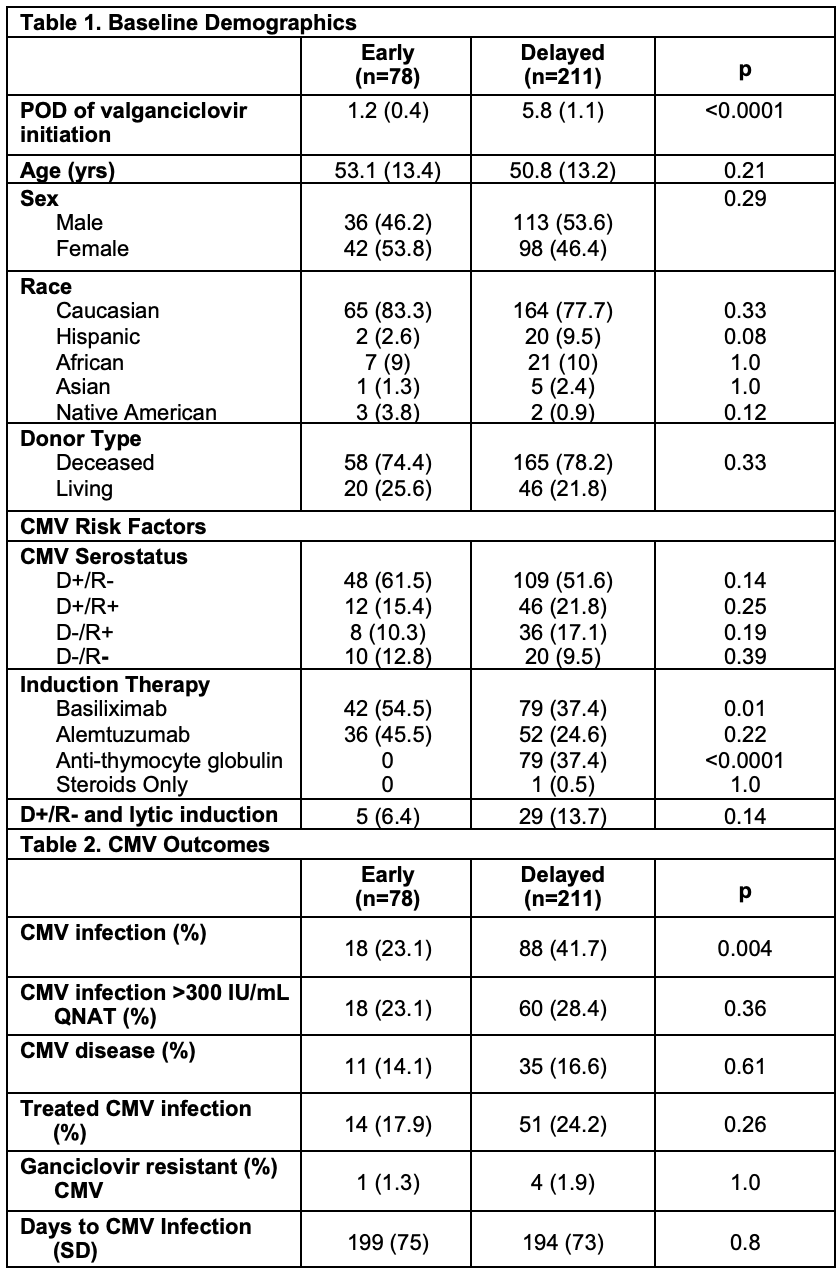
*Purpose: The purpose of this study is to determine the effect of delaying cytomegalovirus (CMV) prophylaxis to day of discharge or post-operative day (POD) 7 on the incidence of CMV infection in high-risk kidney transplant recipients.
*Methods: A retrospective, single-center review was conducted on adult kidney transplant recipients transplanted between 1/1/2014 and 12/31/2019 at high-risk of CMV infection defined as CMV D+/R- or use of lytic induction. All patients received valganciclovir (VGCV) prophylaxis for 6 months. Patients were grouped by early initiation of VGCV by POD3 or delayed initiation on or after POD4. The primary outcome was the incidence of CMV infection defined as detection of CMV in the blood by quantitative nucleic acid amplification test (QNAT) within 12 months post-transplant. The CMV PCR assay increased in sensitivity during the study period which allowed detection of lower levels of viremia in a subset of the delayed group. Secondary outcomes included time to CMV infection, incidence of CMV infection with QNAT>300IU/mL, CMV disease, treated CMV infection, and cost of VGCV during the first week post-transplant.
*Results: There were 78 patients in the early group and 211 patients in the delayed group (Table 1). On average, VGCV was initiated POD 1.2 in the early group and POD 5.8 in the delayed group. Patients in the early group were more likely to receive basiliximab (54.5% vs. 37.4%, p=0.01), while patients in the delayed group were more likely to receive anti-thymocyte globulin (0% vs 37.4%, p=<0.0001). The incidence of CMV infection in the early group was lower than the delayed group (23.1% vs 42.7%, p=0.004) with no difference in average time to CMV infection (199 vs 194 days, p=0.8). There was no difference in the incidence of CMV infection with QNAT>300 IU/mL (23.1% vs 28.4%, p=0.36), CMV disease (14.1% vs 16.6%, p=0.61), or treated CMV infection (17.9% vs 24.2%, p=0.26) (Table 2). The average cost of VGCV during the first week of the index hospitalization was $161 in the early group compared to $13 in the delayed group (p=0.004).
*Conclusions: A higher incidence of CMV infection was identified in the delayed group compared to the early group which may be due to the increased sensitivity of the CMV PCR assay. No differences in CMV infection QNAT>300IU/mL, CMV disease, or treated CMV infection were identified despite a more aggressive induction regimen in the delayed group. Delaying initiation of CMV prophylaxis with VGCV may decrease medication cost during the index hospitalization without negatively impacting clinically relevant infectious outcomes.
To cite this abstract in AMA style:
Hutchins A, Leick M, Keck M, McMullen JS. Early versus Delayed Cytomegalovirus Prophylaxis in High-Risk Kidney Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/early-versus-delayed-cytomegalovirus-prophylaxis-in-high-risk-kidney-transplant-recipients/. Accessed March 9, 2026.« Back to 2022 American Transplant Congress