Early Results from USHER: A Pilot Trial of Transplanting HCV-Infected Hearts into HCV-Negative Recipients Followed by Anti-Viral Therapy
UPENN, Philadelphia.
Meeting: 2018 American Transplant Congress
Abstract number: 599
Keywords: Donation, Graft function, Heart transplant patients
Session Information
Session Name: Concurrent Session: Late Breaking
Session Type: Concurrent Session
Date: Tuesday, June 5, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 4:42pm-4:54pm
Presentation Time: 4:42pm-4:54pm
Location: Room 3AB
Background: In 2016, over 600 hearts from deceased donors with active or prior hepatitis C virus (HCV) infection were discarded; most were otherwise good quality, given the donors' profile. USHER is a single-arm trial of orthotopic heart transplantation (OHT) using HCV-infected organs for HCV-negative candidates, followed by elbasvir/grazoprevir post-transplant when recipient HCV is detected (sponsor: Merck; NCT0314674).
Methods: Inclusion criteria include: 40 – 65 years old; waitlisted for OHT. Exclusions include: liver disease; LVAD.
Donor HCV genotyping allowed us to restrict to organs from HCV genotype 1 or 4 donors. USHER target is 10 OHTs.
Results: From 5/1/17-1/17/18, 26 patients were approached, 16 (62%) went to education sessions, 15 (58%) consented for screening and enrolled. Median age 53.5 years (IQR 50, 58); 3 (21%) female; 3 (21%) black race. After waiting a median of 31 days (IQR 17, 57) from UNOS opt-in for HCV-NAT+ donor offers, nine patients had OHT from HCV-genotype-1 donors (median donor age 33 years, IQR 31, 36). Initial recipient HCV levels varied from 25 IU/mL to 40 million IU/mL, but all patients' HCV NAT levels declined rapidly after elbasvir/grazoprevir treatment (Figure). Two patients were cured (SVR-12); 7 have on-treatment undetectable HCV but have not reached SVR timepoint; SVR data will be available June 2018. Five of 9 recipients had ALT elevations post-transplant that resolved in follow-up. All recipients have good allograft function. Serious adverse events included 1) acute kidney injury from early poor cardiac output and pericardial tamponade; the patient requires dialysis at week 12; 2) another had antibody mediated rejection, required ECMO, had acute kidney injury, but has since had good allograft recovery; and 3) another had cellular rejection responsive to oral steroids. None of these were related to HCV or treatment.
Conclusions: Preliminary evidence suggests that HCV-negative candidates who undergo OHT with HCV-infected organs have acceptable early outcomes and respond well to antiviral therapy 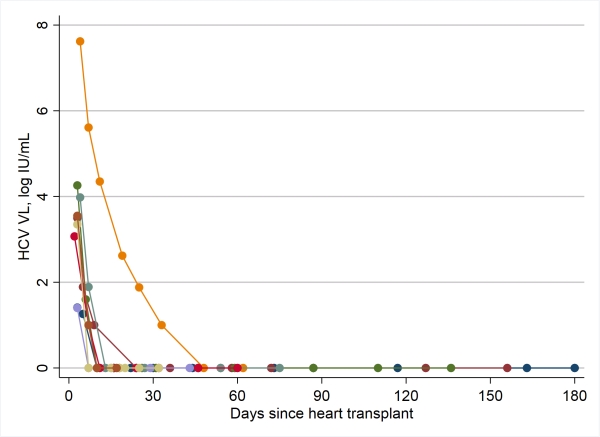
CITATION INFORMATION: McLean R., Reese P. P., Acker M., Abt P., Sicilia A., Hornsby N., Atluri P., Reddy K. R., Blumberg E., Van Deerlin V., Goldberg D. S. Early Results from USHER: A Pilot Trial of Transplanting HCV-Infected Hearts into HCV-Negative Recipients Followed by Anti-Viral Therapy Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
McLean R, Reese PP, Acker M, Abt P, Sicilia A, Hornsby N, Atluri P, Reddy KR, Blumberg E, Deerlin VVan, Goldberg DS. Early Results from USHER: A Pilot Trial of Transplanting HCV-Infected Hearts into HCV-Negative Recipients Followed by Anti-Viral Therapy [abstract]. https://atcmeetingabstracts.com/abstract/early-results-from-usher-a-pilot-trial-of-transplanting-hcv-infected-hearts-into-hcv-negative-recipients-followed-by-anti-viral-therapy/. Accessed March 6, 2026.« Back to 2018 American Transplant Congress
