Early Outcomes of Single Dose Eculizumab for Abo-Incompatible Living Donor Renal Transplantation
UI Health, Chicago, IL
Meeting: 2022 American Transplant Congress
Abstract number: 1689
Keywords: Graft function, Kidney transplantation, Living donor, Rejection
Topic: Clinical Science » Kidney » 38 - Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Hall C
*Purpose: Use of ABO-incompatible (ABOi) donors increases access to living donor renal transplantation (LDRT). Conventional desensitization with plasmapheresis (PLEX) allows safe ABOi transplantation when pre-transplant anti-ABO antibody titers are <1:8. In 2017, we reported excellent patient and allograft outcomes when a 9-week eculizumab (ECU) course was used in place of PLEX for ABOi LDRT. We recently abbreviated our protocol to a single, pre-operative dose of ECU 900 mg (SDE protocol) and report short term outcomes in a cohort of 9 patients.
*Methods: Regardless of pre-transplant ABO titer, all patients received a single 900 mg dose of ECU prior to transplantation, along with rabbit anti-thymocyte globulin induction (rATG), tacrolimus, mycophenolate, and 1-month prednisone taper to 10 mg daily. No PLEX was used. Patients received meningococcal, pneumococcal, and Haemophilus influenzae type b vaccines prior to ECU, and meningitis prophylaxis with penicillin VK or levofloxacin for 4 weeks after ECU.
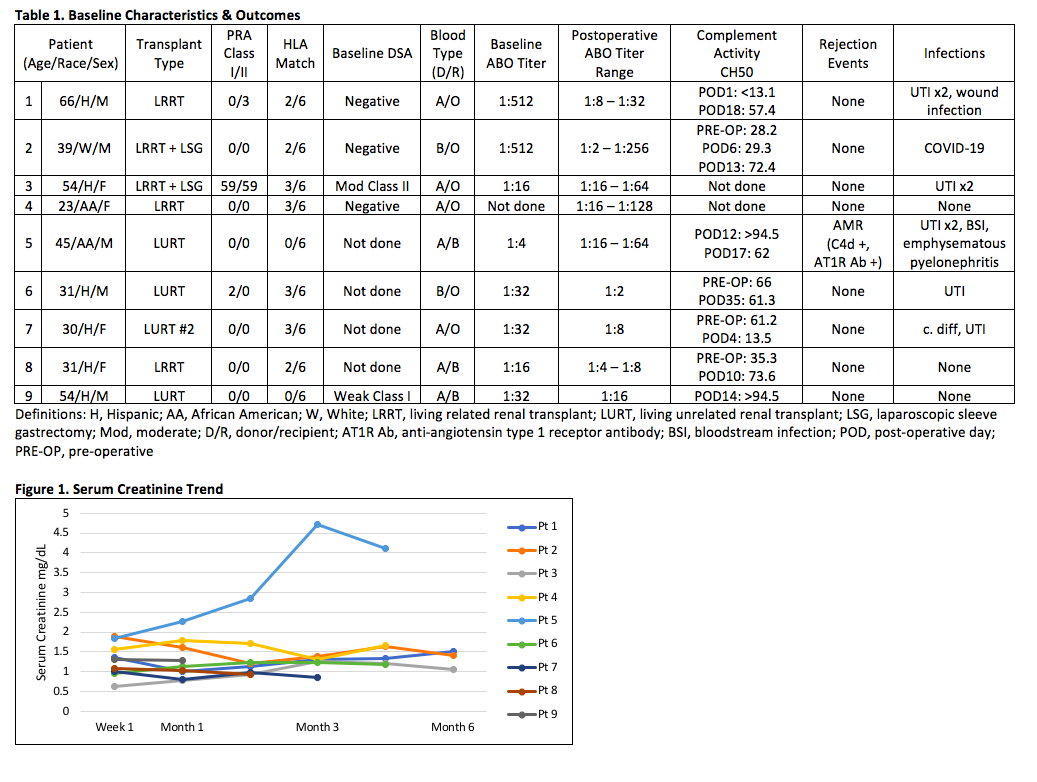
*Results: Six of 9 patients were Hispanic with median age of 39 years. All but one patient had low PRA <10% (Table 1). Eight patients had no rejection episodes and excellent allograft function at the latest follow up (Figure 1). Patient 5 developed biopsy proven antibody mediated rejection (AMR) in the setting of subtherapeutic tacrolimus trough levels 2 weeks post-transplant. The same patient subsequently developed severe pyelonephritis following treatment with bortezomib, rATG, and PLEX. He eventually recovered with worse renal function. Additional infectious outcomes are demonstrated in Table 1, which were most commonly UTI.
*Conclusions: Early outcomes following a single dose of ECU 900 mg for ABOi LDRT showed excellent allograft function and patient outcomes without the need for PLEX and anti-ABO titer monitoring. Infectious complications were mild in most cases.
To cite this abstract in AMA style:
Heagler K, Campara M, Cocco PDi, Gaitonde S, Tang I, Alvarez JAlmario, Spaggiari M, Benken J, Muran CS, Valdepenas B, Pierce D, Tzvetanov I, Benedetti E. Early Outcomes of Single Dose Eculizumab for Abo-Incompatible Living Donor Renal Transplantation [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/early-outcomes-of-single-dose-eculizumab-for-abo-incompatible-living-donor-renal-transplantation/. Accessed February 27, 2026.« Back to 2022 American Transplant Congress