Early Clinical Experience With Alvr109, A Partially Hla-matched Sars-cov-2 Specific T-cell Therapy, In Immunocompromised Patients With Covid-19
1University of Pittsburgh, Pittsburgh, PA, 2UPitt, Pittsburgh, PA, 3Allovir, Waltham, MA
Meeting: 2022 American Transplant Congress
Abstract number: 9011
Keywords: COVID-19, Lung infection, T cells
Topic: Basic & Clinical Science » Basic & Clinical Science » 73 - COVID-19
Session Information
Session Name: Late Breaking: COVID-19
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 2:00pm-3:00pm
 Presentation Time: 2:40pm-2:50pm
Presentation Time: 2:40pm-2:50pm
Location: Hynes Room 310
*Purpose: Therapies for COVID-19 in immunocompromised (IC) patients (pts), including transplant (tx) pts, are limited. We describe our experience with ALVR109, an allogeneic, partially HLA-matched T-cell product, given through emergency investigational new drug (eIND) application to 4 consecutive IC pts with protracted COVID-19.
*Methods: To measure SARS-CoV-2 viral loads, SARS-2 RNA was quantified by RT-PCR (N gene) in plasma and saliva. ALVR109 was manufactured for Allovir at Baylor College of Medicine.
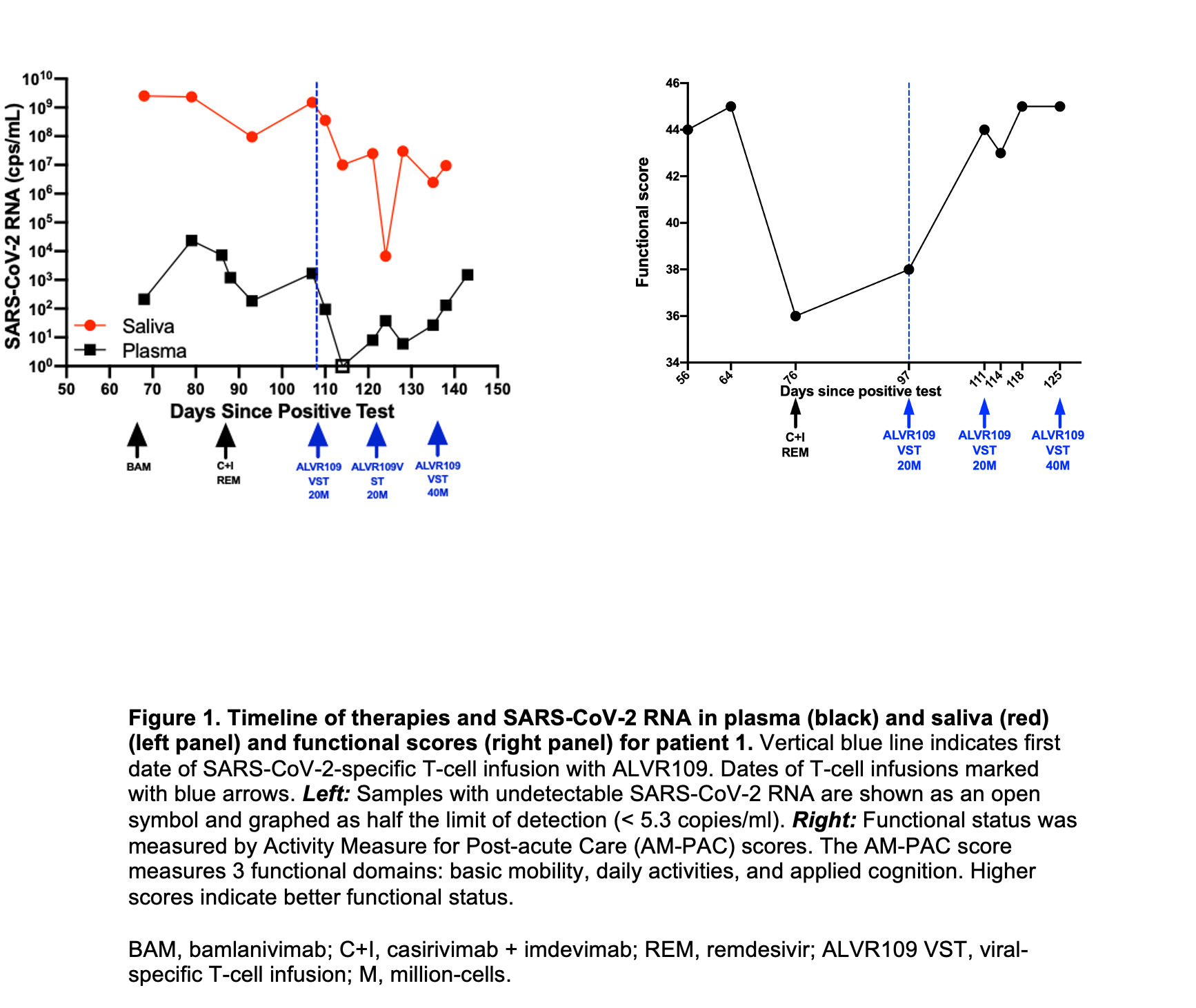
*Results: Between May and October 2021, ALVR109 was given to 4 IC pts with COVID-19 (details in Table 1). 2 pts had lymphoma (1 post auto-tx) and 2 had lung tx. All pts had SARS-CoV-2 RNA detected in plasma (viremia) in the weeks leading up to ALVR109 administration. Infusions (20-40 million cells (MC) per dose) were well-tolerated with no adverse events. Prior to ALVR109, pts 1 and 3 had progressive COVID-19 and ongoing SARS-CoV-2 viremia despite monoclonal antibodies (mABs) and remdesivir. Following ALVR109 administration both patients had a decrease in viremia with marked clinical improvement in pt 1, but both eventually died from their underlying disease. Viral loads (plasma/saliva) and functional scores for pt 1 are shown in the figure. Autopsy of pt 3 showed no evidence of SARS-CoV-2 infection by lung in-situ hybridization (ISH). Pts 2 and 4 received ALVR109 as adjunctive therapy to mABs and remdesivir; viremia continued to decline following ALVR109 and both pts survived and were discharged home.
*Conclusions: This initial experience suggests a potential role of ALVR109 in the treatment of IC and tx pts with COVID-19. SARS-CoV-2-specific T-cells appear to be safe and may control viremia in IC pts. Larger studies are needed to confirm this observation, define the best candidates for ALVR109, and determine optimal timing of administration.
| Pt | Demo | Condition | Days of illness pre-ALVR109 | Peak SARS-CoV-2 viremia(cps/mL) | Rx pre ALVR109 (days) | # ALVR109 infusions (dose) | Treatment response and outcomes |
| 1 | 55F | DLBCL | 97 | 23680 | BAM (91)C+I (21)REM (21) | 3 (20MC x2, then 40MC) | Viremia x months, persisted with mABs/REM. After 2x ALVR109: viremia undetectable (UD), clinical improvement. Died from cancer. |
| 2 | 56M | Auto-tx | 245 | 245 | REM (12)C+I (11) | 3 (20MC) | Viremia x months, cleared with mABs/REM. After 3 x ALVR109: viremia remained UD, clinically improved, no relapse. |
| 3 | 61M | Lung tx | 34 | 723 | REM (33)STV (34) | 1 (20MC) | Viremia, critical illness after REM and STV. Viremia suppressed post ALVR109. Died of organ failure. Lung stain for SARS-CoV-2 at autopsy negative. |
| 4 | 72M | Lung tx | 15 | 1940 | REM (12)C+I (11) | 1 (20MC) | Viremia cleared and clinically improved with REM and C+I; critically ill but improvement continued after ALVR109. Discharged home, no relapse |
To cite this abstract in AMA style:
Haidar G, Jacobs J, Hughes K, McCormick K, Naqvi A, Agha M, Bogdanovich T, Johnson B, Kozar J, Lendermon E, Marino C, Raptis A, Silveira F, Marshall W, Miller M, Atillasoy E, Mellors J. Early Clinical Experience With Alvr109, A Partially Hla-matched Sars-cov-2 Specific T-cell Therapy, In Immunocompromised Patients With Covid-19 [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/early-clinical-experience-with-alvr109-a-partially-hla-matched-sars-cov-2-specific-t-cell-therapy-in-immunocompromised-patients-with-covid-19/. Accessed March 2, 2026.« Back to 2022 American Transplant Congress