DSA Monitoring in Kidney Transplant Recipients
1Medstar Georgetown Transplant Institute, Georgetown University, Washington, DC
2Clinical Laboratory and Histocompatibility, Georgetown University, Washington, DC.
Meeting: 2015 American Transplant Congress
Abstract number: A292
Keywords: Antibodies, Graft failure
Session Information
Session Name: Poster Session A: Late Breaking
Session Type: Poster Session
Date: Saturday, May 2, 2015
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Exhibit Hall E
Introduction: The development of DSA monitoring is a new technique to detect antibodies in kidney transplant recipients. Data has shown that development of DSA post kidney transplantation is associated with worst outcomes. Methods: We began performing DSA monitoring protocol on October 26, 2012. This analysis includes patients up to January 4, 2014 and one year of follow up. 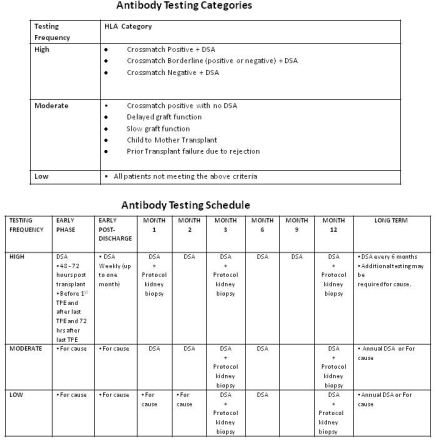 Results: 199 kidney transplant were performed at our transplant center during this period of time and 167 patients completed the DSA monitoring protocol. There was a 11.9% rate of dnDSA. Of the patients that developed dnDSA, 20% were class I, 60% were class II and 20% were both class I and II. 75% of patients developed either cellular or humoral rejection and 35% of patients lost their graft. Conclusions: The one year rejection and graft loss rate after the development of dnDSA is much higher than the national average. Therefore treatment protocols for dnDSA need to be established and implemented.
Results: 199 kidney transplant were performed at our transplant center during this period of time and 167 patients completed the DSA monitoring protocol. There was a 11.9% rate of dnDSA. Of the patients that developed dnDSA, 20% were class I, 60% were class II and 20% were both class I and II. 75% of patients developed either cellular or humoral rejection and 35% of patients lost their graft. Conclusions: The one year rejection and graft loss rate after the development of dnDSA is much higher than the national average. Therefore treatment protocols for dnDSA need to be established and implemented.
To cite this abstract in AMA style:
Pean F, Cooper M, Verbesey J, Gilbert A, Javaid B, Mohammed R, Timofeeva O, Moore J, Cruz K, Ghasemian R, Fishbein T, Grafals M. DSA Monitoring in Kidney Transplant Recipients [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/dsa-monitoring-in-kidney-transplant-recipients/. Accessed February 16, 2026.« Back to 2015 American Transplant Congress
