Donor-Specific Antibody Test as a Screening Tool for Stable Long-Term Kidney Transplant Recipients
1Department of Nephrology, Madison, WI, 2Department of Surgery, Madison, WI
Meeting: 2020 American Transplant Congress
Abstract number: C-091
Keywords: HLA antibodies, Kidney transplantation, Outcome, Screening
Session Information
Session Name: Poster Session C: Kidney Complications: Immune Mediated Late Graft Failure
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Donor-specific antibody (DSA) is an established biomarker predicting antibody mediated rejection (AMR), but at substantial cost. The utility of DSA test as a screening tool among stable long-term kidney transplant recipients (KTR) with no previous rejection is unknown.
*Methods: We included KTR from 2013-2018 with no prior rejection or positive DSA, with at least one subsequent DSA test and had negative DSA test at least 3-yrs post-transplant. Positive DSA was defined by anti-HLA median fluorescence intensity [MFI] >100. We examined: a) the probability of subsequently developing positive DSA, b) the prevalence of rejection in a biopsy performed within 3 months of the first positive DSA test, and c) risk of death-censored allograft failure for negative and positive DSA results.
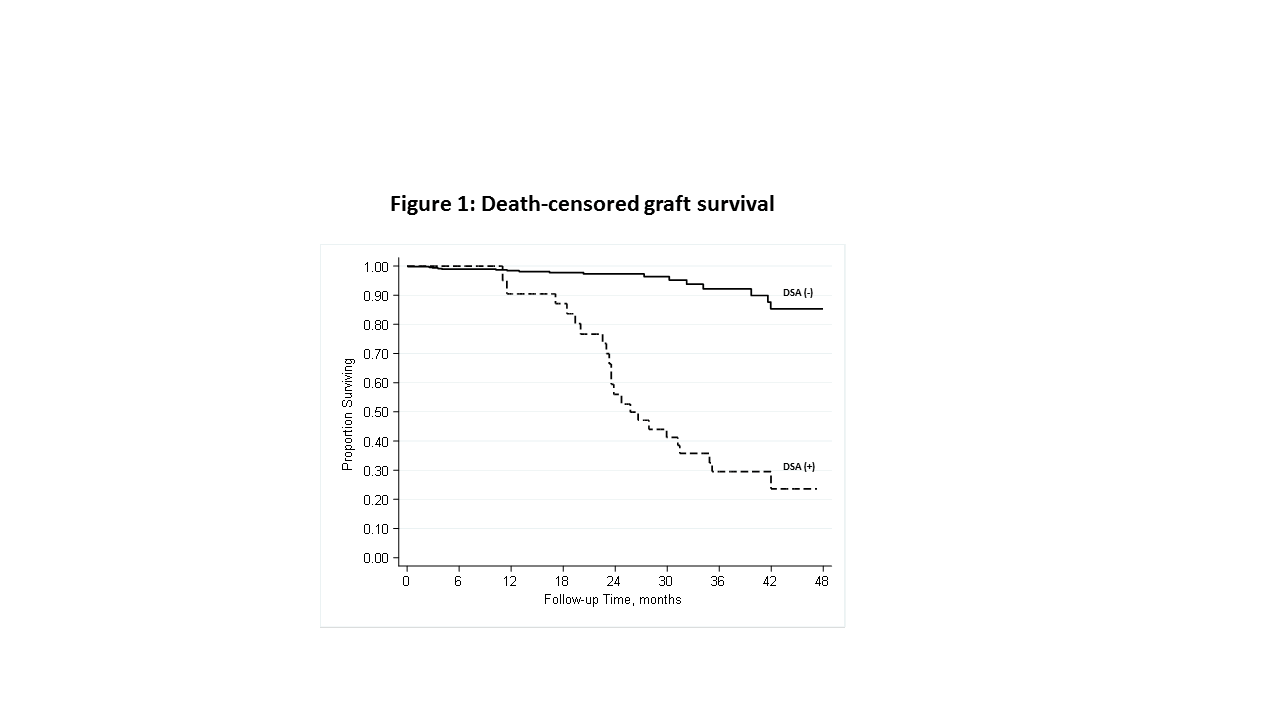
*Results: A total of 2284 KTR recipients had 5053 subsequent DSA tests performed during the study period (median follow-up of 2-yrs). Mean age at transplant was 49 years, 14% are nonwhite, 43% were female, 46% living-donor recipients, 19% had prior transplant, and 19% had diabetes. A total of 555 recipients (24.3%) developed a positive DSA during follow-up. Biopsy results were available for 122 of these KTR with a positive DSA, and 32 (26.2%) were positive for rejection, table 1. Clinical follow-up was available for 467 KTR. A total of 40 graft failures occurred during a median follow-up of 2.5-yrs. A positive DSA was associated with a significantly higher incidence of death-censored graft failure (p<0.001) in analyses considering a positive DSA test as a time-varying predictor, figure 1. This association remained after adjustment for potential confounders.
*Conclusions: Among stable KTR with no prior positive DSA or rejection, 24% develop positive DSA in the subsequent 2 years. A positive DSA had poor correlation with rejection on subsequent biopsy (26% positive). A positive DSA test, however, strongly predicted subsequent death-censored graft failure. Developing criteria to identify those stable KTR at highest likelihood of benefitting from continued screening is critical for cost-effective application.
| Recipients | Tests | Biopsies within 3 months of + DSA | ||||
| Total | Positive DSA (%) | Total | Positive DSA (%) | Total | Rejection (%) | Mediantime to rejection (IQR), days |
| 2284 | 555 (24.3) | 5053 | 1414 (28.0) | 122 | 32 (26.2) | 290 (103, 377) |
To cite this abstract in AMA style:
Mohamed M, Hidalgo L, Parajuli S, Garg N, Aziz F, Swanson K, Jochaim E, Mandelbrot D, Djamali A, Astor B. Donor-Specific Antibody Test as a Screening Tool for Stable Long-Term Kidney Transplant Recipients [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/donor-specific-antibody-test-as-a-screening-tool-for-stable-long-term-kidney-transplant-recipients/. Accessed February 20, 2026.« Back to 2020 American Transplant Congress