Donor-Derived Cell-Free DNA Can Differentiate Rejection versus Other Causes of Pancreas Transplant Dysfunction
1Transplant Institute, NYU Langone Health, New York, NY, 2CareDx, Brisbane, CA
Meeting: 2021 American Transplant Congress
Abstract number: 1216
Keywords: Graft function, Kidney/pancreas transplantation, Pancreatitis, Rejection
Topic: Clinical Science » Pancreas » Pancreas and Islet: All Topics
Session Information
Session Name: Pancreas and Islet: All Topics
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Assessment and early detection of allograft injury in simultaneous pancreas and kidney (SPK) transplant recipients has clear benefits for optimizing treatment and intervention. Donor-derived cell-free DNA (dd-cfDNA) is an established biomarker for surveillance of kidney transplant recipients and an increasing body of evidence supports its utility in SPK transplantation.
*Methods: SPK patients were monitored prospectively with dd-cfDNA (AlloSure, CareDx Brisbane), initiated at the discretion of the treating physician. dd-cfDNA was drawn concomitantly with standard of care laboratory testing including serum creatinine, amylase, lipase, DSA and BK PCR. Pancreatic graft dysfunction was defined by elevations in serum amylase and lipase or dysregulated glucose control.
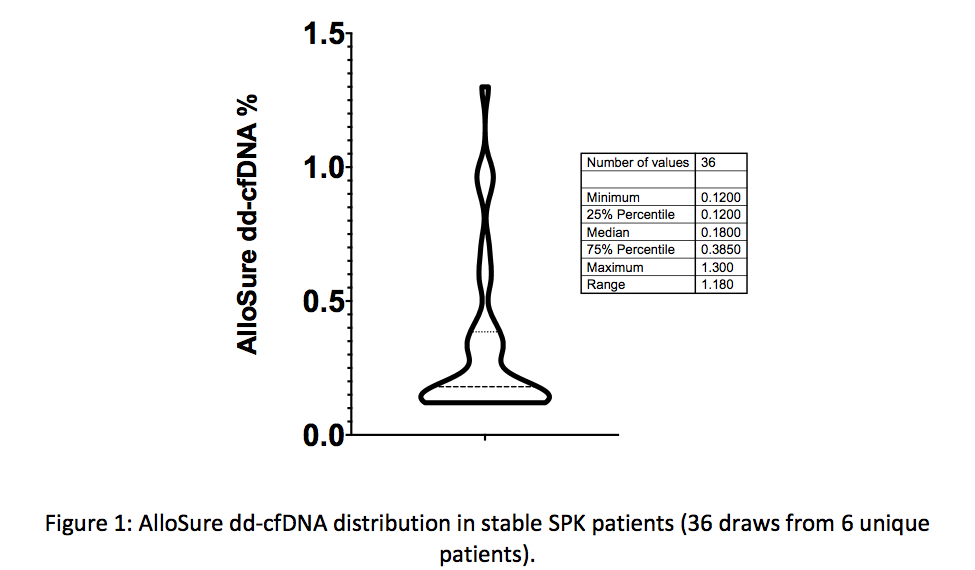
*Results: A total of 9 SPK patients were identified with a total of 49 AlloSure dd-cfDNA results. Median patient age was 48 years (range 26-56). There were 5 females (56%) recipients, 2 Caucasian (22%), 3 Black (33%) and 4 Hispanic (44%) recipients. 6 SPK patients had stable graft function and 3 SPK patients developed pancreatic graft dysfunction. The median AlloSure dd-cfDNA level in stable SPK patients was 0.18% (IQR 0.12-0.39%, Figure 1). One SPK recipient with stable pancreatic enzymes had an AlloSure dd-cfDNA level of 1.3% in the setting of sub-therapeutic tacrolimus levels, which normalized with titration of immunosuppression. Of the 3 SPK recipients with pancreatic graft dysfunction, 1 patient had an AlloSure dd-cfDNA level of 3.5% associated with clinical allograft rejection. AlloSure dd-cfDNA levels decreased to 0.14% within 1 month of empiric treatment. The remaining 2 SPK recipients with graft dysfunction had paired AlloSure dd-cfDNA levels of 0.42% and 0.52%. These patients were subsequently diagnosed with a bowel obstruction and partial splenic vein thrombosis as the cause for graft dysfunction.
*Conclusions: In stable SPK recipients, AlloSure dd-cfDNA levels remain low during the post-transplant course. In this case series, AlloSure dd-cfDNA was able to differentiate allograft injury associated with rejection from alternative etiologies of graft dysfunction. Elevated dd-cfDNA levels associated with rejection and sub-therapeutic immunosuppression decreased following intervention. Further studies are required to further define reference values and response to intervention.
To cite this abstract in AMA style:
Ali N, Miles J, Lewis ZStewart. Donor-Derived Cell-Free DNA Can Differentiate Rejection versus Other Causes of Pancreas Transplant Dysfunction [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/donor-derived-cell-free-dna-can-differentiate-rejection-versus-other-causes-of-pancreas-transplant-dysfunction/. Accessed February 19, 2026.« Back to 2021 American Transplant Congress