Distinct Phenotype and Function of Plasmacytoid Dendritic Cells from Kidney Allografts in Tolerant Recipients.
1Center for Transplantation Sciences, Massachusetts General Hospital, Boston, MA
2Department of Surgery, Massachusetts General Hospital, Boston, MA
3Department of Pathology, Massachusetts General Hospital, Boston, MA.
Meeting: 2016 American Transplant Congress
Abstract number: 205
Keywords: Graft acceptance, Kidney, Kidney transplantation
Session Information
Session Name: Concurrent Session: Innate Immunity in Rejection and Tolerance: Animal Models
Session Type: Concurrent Session
Date: Monday, June 13, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 3:42pm-3:54pm
Presentation Time: 3:42pm-3:54pm
Location: Room 313
BACKGROUND: Cellular infiltrates in kidney allografts may be relevant to the induction or maintenance of regulatory tolerance. Here we examined the phenotype, anatomical distribution and function of plasmacytoid dendritic cells in kidney allografts from recipients made tolerant by mixed chimerism protocols (nonhuman primates, NHP) or by natural mechanisms (mice).
METHODS: Plasmacytoid dendritic cells (pDC) populations in NHPs rendered tolerant to heart and kidney allografts using a mixed-chimerism protocol and in mice with spontaneously accepted kidney allografts were analyzed using flow cytometry and immunohistochemistry. Bone marrow, naïve and accepted kidney pDCs were compared for differences in pDC cell markers. pDCs were isolated from these tissues and cultured with naïve T-cells, IL-2, TGFβ to test for Foxp3 induction.
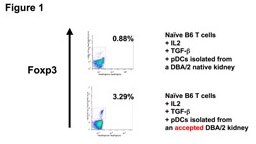
RESULTS: We observed distinct Treg-rich organized lymphoid structures (TOLS) in spontaneously accepted kidneys in mice and tolerant NHP. These had prominent Foxp3+ cells and pDCs. pDCs in the tolerant NHP kidneys were found to be BDCA2hi (pDC marker in NHP) in contrast to bone marrow pDCs in naïve NHPs, which were BDCA2lo. No pDCs were detected in native kidneys. Tolerant murine kidney pDCs were Siglec-Hhi (pDC marker in mice), whereas naïve kidney pDCs were Siglec-Hlo. Immunohistochemistry revealed gradual localization of pDCs around TOLS over time in both NHPs and mice. Isolated tolerant kidney pDCs cultured with naïve T cells, IL-2, and TGFβ were able to convert T-cells into Foxp3+ Tregs with greater potency than pDCs isolated from native kidney (Fig. 1).
CONCLUSIONS: We have identified a unique subset of pDCs that traffic to a distinct location in accepted kidney allografts and that are capable of promoting the expansion of Tregs. These findings suggest that these unique pDCs play an important role in inducing tolerance of kidney allografts across species.
CITATION INFORMATION: Oh N, Yang C, Tonsho M, Ndishabandi D, Russell P, Colvin R, Madsen J, Alessandrini A. Distinct Phenotype and Function of Plasmacytoid Dendritic Cells from Kidney Allografts in Tolerant Recipients. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Oh N, Yang C, Tonsho M, Ndishabandi D, Russell P, Colvin R, Madsen J, Alessandrini A. Distinct Phenotype and Function of Plasmacytoid Dendritic Cells from Kidney Allografts in Tolerant Recipients. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/distinct-phenotype-and-function-of-plasmacytoid-dendritic-cells-from-kidney-allografts-in-tolerant-recipients/. Accessed February 25, 2026.« Back to 2016 American Transplant Congress
