Direct Comparison of Clinical Outcomes with Belatacept-Based Regimens to Calcineurin Inhibitors Among DCD and/or High KDPI Kidney Transplant Recipients
1Internal Medicine, Nephrology, University of Texas Medical Branch at Galveston, Galveston, TX, 2Surgery, University of Texas Medical Branch, Galveston, TX, 3University of Texas Medical Branch, Houston, TX, 4Surgery, University of Texas Medical Branch at Galveston, Galveston, TX, 5University of Texas Medical Branch, Galveston, TX
Meeting: 2022 American Transplant Congress
Abstract number: 1705
Keywords: Calcineurin, Co-stimulation, Immunosuppression, Rejection
Topic: Clinical Science » Kidney » 38 - Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Belatacept, an inhibitor of the T-cell costimulatory pathway, can be used in recipients of Donation after Cardiac Death (DCD) and high Kidney Donor Profile Index (KDPI) renal allografts when there is concern for calcineurin inhibitor (CNI) nephrotoxicity. Data are sparse directly comparing clinical outcomes with Belatacept-based regimens to CNI in this at-risk group. We herein set forth to determine the effect of Belatacept on rejection and clinical outcomes in DCD and/or high KDPI kidney transplant recipients.
*Methods: In this single-center retrospective study, we included patients who underwent kidney transplantation with DCD and/or high KDPI (≥85%) allografts between 2014 to 2021. The primary endpoint was biopsy-proven antibody mediated (ABMR) and/or T-cell mediated rejection (TCMR). Secondary endpoints included hospital length of stay (LOS), denovo donor-specific antibodies (dnDSAs), and serum creatinine concentration at 3- and 12-months. Patients were categorized into CNI- and Belatacept-groups. Patients with DGF preferentially received Belatacept.
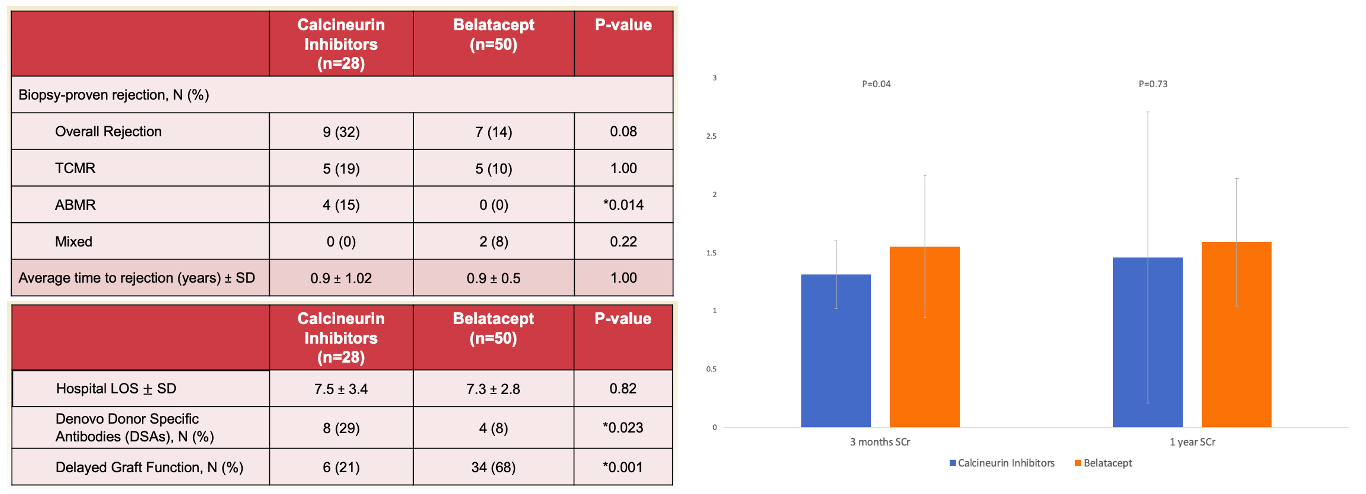
*Results: A total of 78 patients were included. The CNI group included 28 patients (54.5±13.8 years, 43% females) and Belatacept group included 50 patients (51.5±12.4 years, 40% females). Thymoglobulin was used as the induction agent in 100% and 92% of the CNI and Belatacept groups, respectively. Although the incidence of overall biopsy-proven rejection was not statistically different between the groups: (CNI, 32.1%; Belatacept, 14%, p=0.08), ABMR was significantly lower among the Belatacept vs. CNI groups (0% vs. 15%, p=0.014). There was significantly lower incidence of dnDSA among the Belatacept vs. CNI groups (8% vs. 29%, p=0.023) (Figure).
*Conclusions: The use of Belatacept in this immunologically increased risk group of DCD and high KDPI transplants had favorable clinical and rejection outcomes compared to CNI-based regimens, with less ABMR and less dnDSA.
To cite this abstract in AMA style:
Rizvi A, Kueht M, Hussain S, Fair J, Ramirez R, Gamilla-Crudo A, Mujtaba M. Direct Comparison of Clinical Outcomes with Belatacept-Based Regimens to Calcineurin Inhibitors Among DCD and/or High KDPI Kidney Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/direct-comparison-of-clinical-outcomes-with-belatacept-based-regimens-to-calcineurin-inhibitors-among-dcd-and-or-high-kdpi-kidney-transplant-recipients/. Accessed March 7, 2026.« Back to 2022 American Transplant Congress