Development of an Integrated Transplant Data Mart for Customized Data Reporting.
Emory University, Atlanta.
Meeting: 2016 American Transplant Congress
Abstract number: C76
Keywords: Graft failure, Morbidity, Mortality, Surgical complications
Session Information
Session Name: Poster Session C: Economics, Public Policy, Allocation, Ethics
Session Type: Poster Session
Date: Monday, June 13, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Background: Transplant centers have a large amount of clinical data from multiple data sources that are utilized for quality tracking of clinical outcomes, regulatory reports, and research. Merging and combining these varied data sources are resource- and time-intensive.
Methods: Using a multidisciplinary team of clinicians, information technologists, and data analysts, our institution created a consolidated transplant data mart (TDM). The TDM integrates multiple sources of clinical data (clinical data warehouse data, human leukocyte antigen typing results, supplemental specimen information on consented research subjects, pre-transplant data, Laboratory Information Systems data, etc). Data sources are integrated into a single, common Oracle Business Intelligence database and most data are updated nightly, creating a robust database with a <24-hour lag. Oracle Business Intelligence was placed on top of the TDM to support end-user access, creating a user-friendly interface that allows for reporting of patient demographics, longitudinal measures of health status, and program-level outcomes.
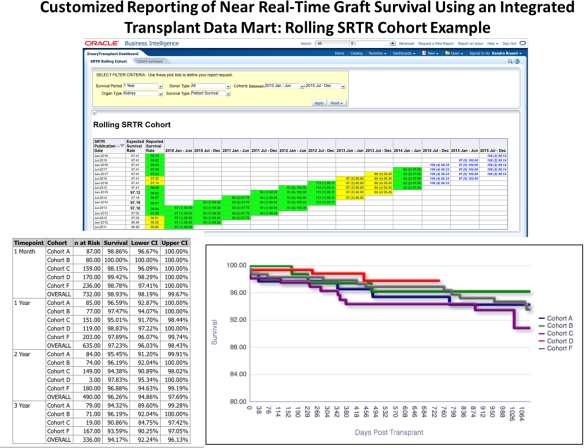
Results: A total of 8,393 transplant recipients (including 4,452 kidney, 2,016 liver, and 1,949 other solid organ transplant recipients) from 5/14/1970 to present are included. To date, we have created 7 dashboards and 68 reports in the web-based tool, replacing the spreadsheets and graphs that were manually generated on a monthly basis. The TDM reports are interactive and provide up-to-date information about clinical outcomes, such as acute rejection, graft failure, patient death, infection, and hospitalization outcomes. Users also have access to an ad-hoc reporting feature, allowing for investigator-driven analysis. For example, program-level outcomes associated with the Scientific Registry of Transplant Recipients (SRTR) are available to end users in near real-time.
Conclusions: The TDM is a powerful tool, granting access to a wealth of validated data to drive quality improvement in the transplant center. A data mart that links and aggregates data from multiple sources allows providers to describe transplant populations in real time and could help centers improve patient outcomes.
CITATION INFORMATION: Patzer R, Little K, Kirsch J, Weaver J, Adams A. Development of an Integrated Transplant Data Mart for Customized Data Reporting. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Patzer R, Little K, Kirsch J, Weaver J, Adams A. Development of an Integrated Transplant Data Mart for Customized Data Reporting. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/development-of-an-integrated-transplant-data-mart-for-customized-data-reporting/. Accessed March 7, 2026.« Back to 2016 American Transplant Congress
