Development and Validation of a Capillary Blood Mitra-Based Assay for the Quantitative Determination of Advagraf® Concentrations in Transplant Patients
1Astellas Pharma Europe Ltd, Chertsey, United Kingdom, 2Covance Laboratories, Harrogate, United Kingdom, 3Kings College Hospital, London, United Kingdom, 4CHU Rangueil, Toulouse, France, 5Hôpital Paul-Brousse, Villejuif, France, 6Addenbrookes Hospital, Cambridge, United Kingdom, 7Astellas Pharma Europe Ltd, Surrey, United Kingdom
Meeting: 2019 American Transplant Congress
Abstract number: D387
Keywords: Immunosuppression, Kidney transplantation, Liver transplantation, Pharmacokinetics
Session Information
Session Name: Poster Session D: Late Breaking
Session Type: Poster Session
Date: Tuesday, June 4, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Therapeutic drug monitoring of tacrolimus (TAC) requires venepuncture blood samples taken by specialists, requiring patients to attend an outpatient clinic; this can be inconvenient and may affect timing of collection. A new, less-invasive assay based on capillary blood samples (10μL), obtained by a fingerprick that can be self-performed using the MITRA® Microsampler, has been developed and validated. This study compares TAC concentrations determined in venepuncture and fingerprick samples.
*Methods: During routine outpatient follow up visits, 3 paired whole blood samples were collected pre-dose, 1- and 3 hours post-dose from stable adult liver or kidney transplant recipients receiving prolonged-release TAC (ADVAGRAF, Astellas Pharma Europe, BV) -based therapy (NCT03465969). High-performance liquid chromatography tandem-mass spectrometry (HPLC-MS/MS) was used for determination of whole blood TAC concentrations, and the two sampling methods were compared by linear regression and Bland-Altman agreement analyses.
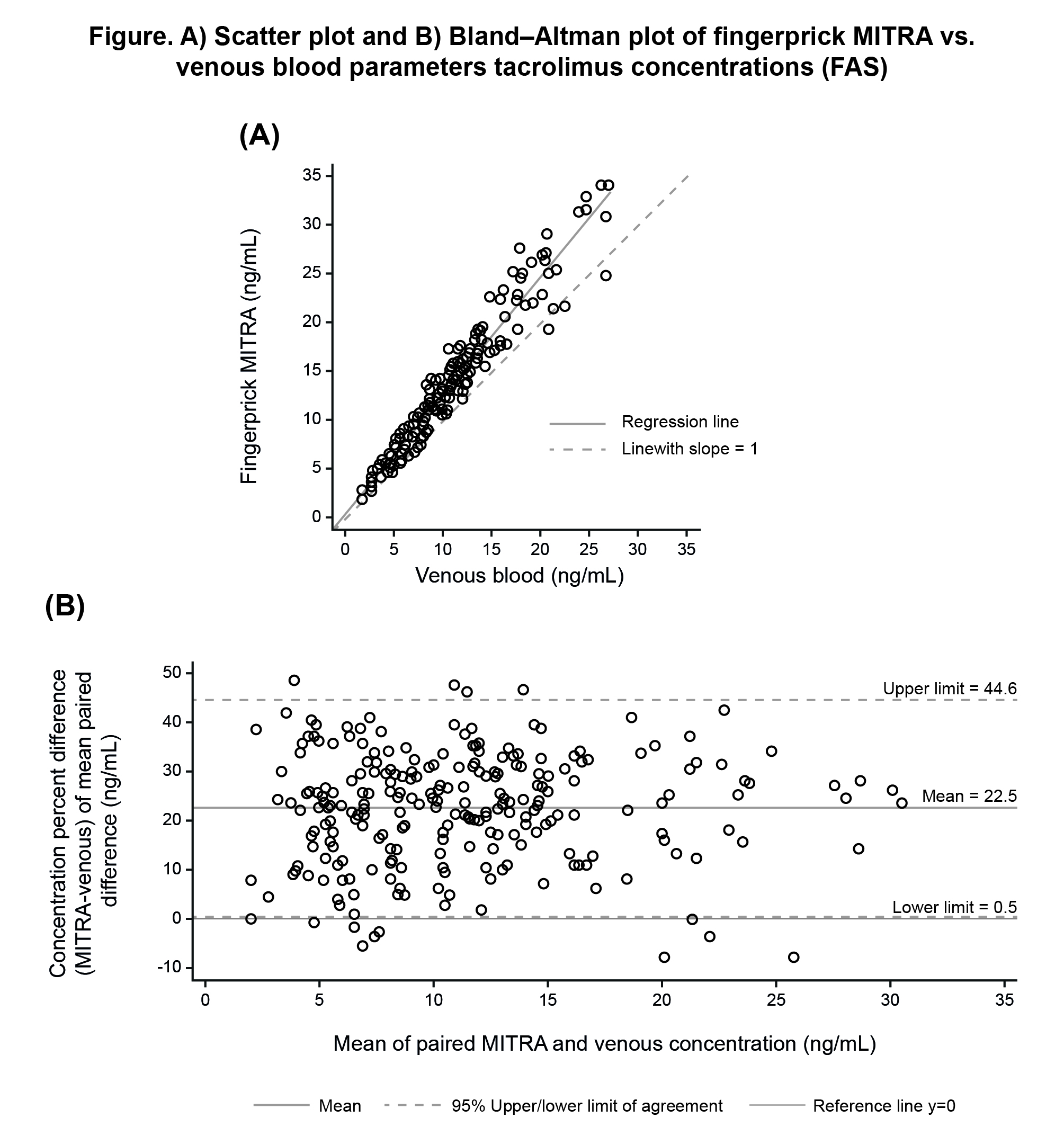
*Results: 82 transplant patients (kidney, n=41; liver, n=41) were studied. Mean±SD age and TAC daily dose were 51±14.8 yrs and 4.6±2.44 mg, respectively. Linear regression analysis showed high correlation between TAC concentrations determined by venepuncture and fingerprick sampling (Pearson’s correlation coefficient [r2], 0.970; Lin’s concordance coefficient, 0.870; slope of the fitted line, >1.0). Bland-Altman agreement analysis showed TAC concentrations in capillary samples were ~22.5% higher on average than in the corresponding venepuncture samples (95% limits of agreement, 0.5% to 44.6%) (Figure).
*Conclusions: This study showed a strong positive correlation between quantitative determination of whole blood TAC concentration by fingerprick MITRA® assay and conventional venepuncture sampling in transplant patients maintained on ADVAGRAF.
To cite this abstract in AMA style:
Undre N, Dawson I, Aluvihare V, Kamar N, Saliba F, Torpey N, Anaokar S, Kazeem G. Development and Validation of a Capillary Blood Mitra-Based Assay for the Quantitative Determination of Advagraf® Concentrations in Transplant Patients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/development-and-validation-of-a-capillary-blood-mitra-based-assay-for-the-quantitative-determination-of-advagraf-concentrations-in-transplant-patients/. Accessed February 24, 2026.« Back to 2019 American Transplant Congress