De Novo or Early Conversion to Everolimus and the Risk of Skin Cancers in Kidney Transplant Recipients: A Trial-Based Linkage Study
1Renal Medicine, Royal Prince Alfred Hospital, Camperdown, Australia
2Kidney Node, Charles Perkins Centre, University of Sydney, Camperdown, Australia.
Meeting: 2018 American Transplant Congress
Abstract number: C74
Keywords: Kidney transplantation, Malignancy
Session Information
Session Name: Poster Session C: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Monday, June 4, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Introduction: Non-melanoma skin cancers (NMSC) are a significant cause of morbidity in kidney transplant (KT) recipients. Choice of immunosuppressive regimen may modify the risk of NMSC after KT. Conversion to sirolimus from CNIs has been shown to reduce the risk of SCCs in high risk pts. However, registry studies and one recent individual patient data (IPD) meta-analysis has shown an excess of deaths in pts on sirolimus. We aimed to compare the incidence of NMSC and death between KT recipients randomised to everolimus (EVL) vs control (CTL) remaining on CNI-based therapy (Rx).
Methods: We included all de novo or early EVL switch RCTs conducted in Australia and NZ KT recipients between 2001-2012. IPD was linked with ANZDATA to capture outcomes of incident NMSC and death. Time-to-first skin cancer was analysed using Kaplan-Meier estimates for different EVL-based Rx [EVL+reduced dose CNI (RD-CNI) or EVL+CNI-withdrawal (CNI-WD)] vs CTL. An adjusted Cox model with random effects was used to determine the overall risks of NMSC and death.
Results: 279 participants (EVL=192, CTL=87) were followed for a median of 9 yrs (IQR 6.8, 9.6). Trial characteristics are shown in Table 1. Of 192 EVL pts, 131 were assigned to EVL+RD-CNI and 61 were assigned to EVL+CNI-WD. The CTL arm consisted of CyA or Tac + MPA or Aza + steroid. 30(15.6%) EVL pts developed new NMSCs (16 SCCs, 14 BCCs) compared with 17(19.5%) CTL pts (8 SCCs, 9 BCCs) (p = 0.4). Compared with CTL, the risk of NMSC was reduced by 55% in the EVL+RD-CNI arm (adj HR 0.45, 95% CI 0.21 – 0.94, p=0.03), but no difference was observed in the EVL+CNI-WD subgroup (adj HR 0.98, 95% CI 0.42-2.27, p=1.0). There was no association between EVL and death (adj HR=1.31 95%CI 0.59–2.91 p=0.5).
Conclusion:In this long-term registry linkage study, EVL+RD-CNI (but not EVL+CNI-WD) was significantly associated with a reduction in new NMSC compared with standard of care. 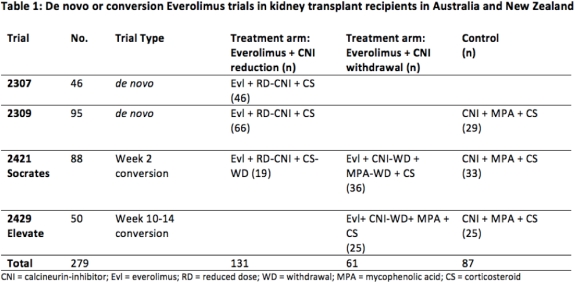
CITATION INFORMATION: Ying T., Wong G., Lim W., Russ G., Pilmore H., Kanellis J., Goodman D., Trevillian P., Campbell S., Suranyi M., Mathew M., Faull R., Masterson R., Walker R., O'Connell P., Chadban S. De Novo or Early Conversion to Everolimus and the Risk of Skin Cancers in Kidney Transplant Recipients: A Trial-Based Linkage Study Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Ying T, Wong G, Lim W, Russ G, Pilmore H, Kanellis J, Goodman D, Trevillian P, Campbell S, Suranyi M, Mathew M, Faull R, Masterson R, Walker R, O'Connell P, Chadban S. De Novo or Early Conversion to Everolimus and the Risk of Skin Cancers in Kidney Transplant Recipients: A Trial-Based Linkage Study [abstract]. https://atcmeetingabstracts.com/abstract/de-novo-or-early-conversion-to-everolimus-and-the-risk-of-skin-cancers-in-kidney-transplant-recipients-a-trial-based-linkage-study/. Accessed February 24, 2026.« Back to 2018 American Transplant Congress
