De Novo DSA in the Setting of Stable Renal Allograft Function Does Not Impair Long-Term Graft Survival
1Surgery, Houston Methodist Hospital, Houston, TX, 2Pathology and Genomic Medicine, Houston Methodist Hospital, Houston, TX
Meeting: 2020 American Transplant Congress
Abstract number: D-055
Keywords: Alloantibodies, HLA antibodies, Kidney, Rejection
Session Information
Session Name: Poster Session D: Kidney Complications: Immune Mediated Late Graft Failure
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: To determine the long-term outcome of renal allograft recipients with de novo DSA (dnDSA) in the setting of stable allograft function.
*Methods: A single-center review of 296 consecutive renal allograft recipients transplanted between January 2008 and March 2010. DSA testing was performed at 3, 6, 9, 12, and every 6 months thereafter. DnDSA were defined as HLA-A, B, Cw, DR, DQ, or DP antibodies directed against the donor, not present pre-transplant. DnDSA had a median florescence index of greater than 2000 and were confirmed by repeated testing. Measurement of C1q binding was not available. Twenty-six recipients were excluded because of pre-transplant DSA (n=22) or initial non-function (n=4).
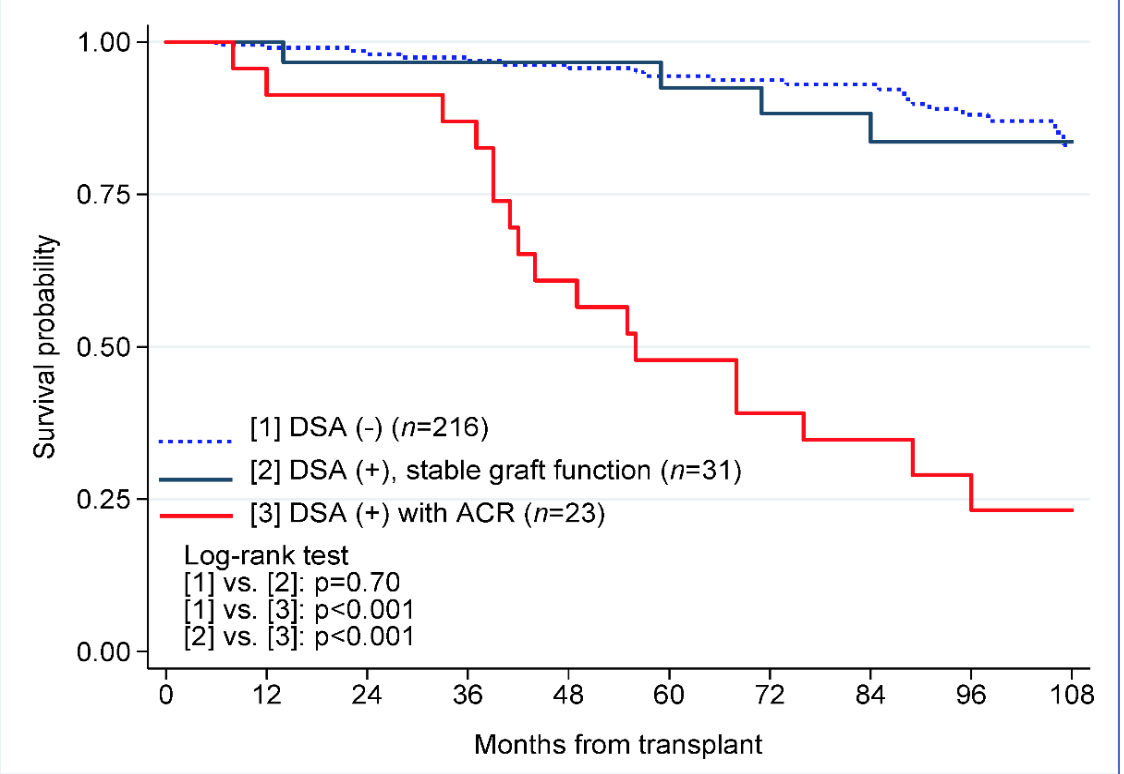
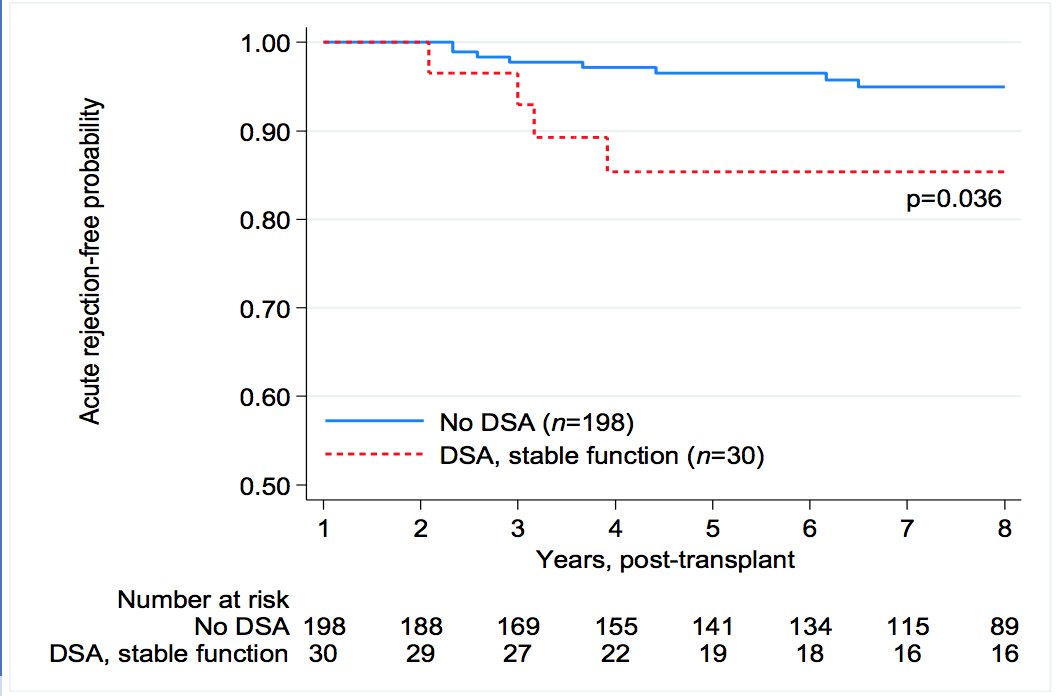
*Results: The study cohort included 216 recipients, 42% female, mean age 50 ± 13 years, 86% primary transplants, 41% living donors, and mean peak PRA of 19±31%. Median follow-up was 76 (range 6-110) months. There were 216 recipients with no DSA (Group 1), 31 recipients with dnDSA and stable graft function (Group 2), and 23 recipients with dnDSA associated with an acute rejection (Group 3). Death censured graft survival was compared between groups 1, 2, and 3 (Figure 1). Median time to first dnDSA for Group 2 was 12 (interquartile range 12-28) versus 17 (7-38) months for Group 3 (p=68). While 81% of Group 2 recipients expressed HLA class II antibodies alone, versus 52% of Group 3 recipients (p=0.02), Group 3 recipients were more likely to express both Class I and II antibodies.(43% versus 10%, p=0.01). A DQ antibody was the most common immunodominant DSA in Group 2 (58%) and Group 3 (74%). Compared to Group 1 recipients, the Group 2 cohort were more likely to suffer a late acute rejection (Figure 2). Mean eGFR (±SD) of Groups 1 and 2 were 62±22 vs. 59±21 at 3 years, 61±22 vs. 62±24 at 5 years, and 57±22 vs.69±26 mls/min at 7 years post-transplantation (p=ns).
*Conclusions: Despite a higher incidence of late acute rejection, development of dnDSA in a recipient with stable graft function resulted in equivalent long-term graft survival and function compared to that of recipients with no dnDSA. In contrast, dnDSA associated with an acute rejection episode generally resulted in long-term graft loss.
To cite this abstract in AMA style:
Knight R, Nguyen DT, Graviss EA, Gaber O. De Novo DSA in the Setting of Stable Renal Allograft Function Does Not Impair Long-Term Graft Survival [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/de-novo-dsa-in-the-setting-of-stable-renal-allograft-function-does-not-impair-long-term-graft-survival/. Accessed February 24, 2026.« Back to 2020 American Transplant Congress