De Novo DSA in the Setting of Stable Allograft Function is a Benign Finding
Houston Methodist Hospital, Houston, TX
Meeting: 2022 American Transplant Congress
Abstract number: 844
Keywords: HLA antibodies, Kidney transplantation, Rejection
Topic: Clinical Science » Kidney » 47 - Kidney Complications: Immune Mediated Late Graft Failure
Session Information
Session Name: Kidney Complications: Immune Mediated Late Graft Failure
Session Type: Poster Abstract
Date: Saturday, June 4, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
*Purpose: We sought to determine the long-term outcome of renal allografts that developed de novo DSA (dnDSA) in the setting of stable renal function.
*Methods: A retrospective single-center review of 605 consecutive renal allograft recipients transplanted between January 2008 and December 2011. DnDSA testing was performed at 3, 6, 9, 12 months, and yearly thereafter. DnDSA were defined as HLA-A, B, Cw, DR, DQ, or DP antibodies directed against the donor, that were not present pre-transplant. All dnDSA had a median florescence index of greater than or equal to 2000 and were confirmed by repeated testing. Measurement of C1q binding was not available. 104 subjects were excluded because of pre-transplant DSA (n=31), follow-up < 6 months (n=26), or limited DSA data (n=104).
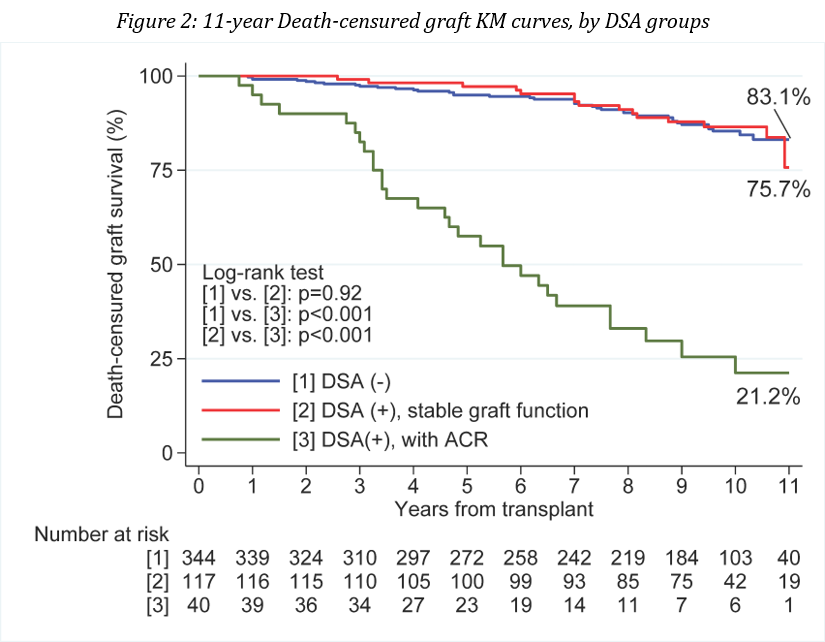
*Results: The study cohort included 501 recipients, with 59% male, mean age 47 ± 13 years, 84% primary transplants and 37% living donors. Median graft survival was 110 (range 9-157) months and the median time to first dnDSA was 12 (range 1-132) months. As shown in Figure 1, death censured graft survival of recipients with no DSA was significantly better than that of dnDSA positive recipients (p<0.001). In Figure 2, dnDSA positive recipients were subdivided into those with stable function and those with dnDSA associated with an acute rejection. There were 344 recipients with no DSA (group 1), 117 recipients with dnDSA and stable graft function (group 2), and 40 recipients with dnDSA associated with an acute rejection (group 3). Death censured graft survival was equivalent between groups 1 and 2, and significantly better than group 3. Seventy-eight percent of group 3 recipients lost their grafts at a median of 56 (range 12-137) months post-transplant. The mean eGFR’s of Groups 1 and 2 were 60±016 vs. 59±16 at one year, 60±19 vs. 57±17 at 5 years, and 59±21 vs 57±21 mls/min at 10 years post-transplantation (p=ns).
*Conclusions: Development of dnDSA in a recipient with stable graft function had no detrimental impact on long-term graft survival, whereas dnDSA associated with an acute rejection mostly resulted in long-term graft loss.
To cite this abstract in AMA style:
Knight RJ, Graviss EA, Nguyen DT, Eagar TN, Yi SG, Podder H, Hobeika M, McMillan R, Gaber AO. De Novo DSA in the Setting of Stable Allograft Function is a Benign Finding [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/de-novo-dsa-in-the-setting-of-stable-allograft-function-is-a-benign-finding/. Accessed March 4, 2026.« Back to 2022 American Transplant Congress