Cytomegalovirus Prophylaxis Following Alemtuzumab Induction in High Risk Renal Transplant Recipients Experiencing Delayed Graft Function
University of Maryland, Baltimore.
Meeting: 2018 American Transplant Congress
Abstract number: B123
Keywords: Dosage, Ganciclovir, Infection
Session Information
Session Name: Poster Session B: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Purpose: There is limited data to support the use of valganciclovir in patients on dialysis. This study assessed the efficacy of valganciclovir 450 mg twice weekly dosing in CMV high risk patients with DGF compared to those without DGF.
Methods: This single center, retrospective, cohort study included first time deceased donor kidney-only transplant recipients from 2007 to 2016 who received alemtuzumab induction and were at high risk for CMV (donor +/recipient -). All patients received a standard immunosuppression (IS) regimen (tacrolimus + mycophenolate + steroid taper) and 6 months of CMV prophylaxis. The primary endpoint was CMV viremia by 12 months. Secondary endpoints included CMV viremia by 3 and 6 months, and CMV syndrome, CMV tissue-invasive disease, CMV resistance, BPAR, graft loss, death, leukopenia, and neutropenia by 12 months.
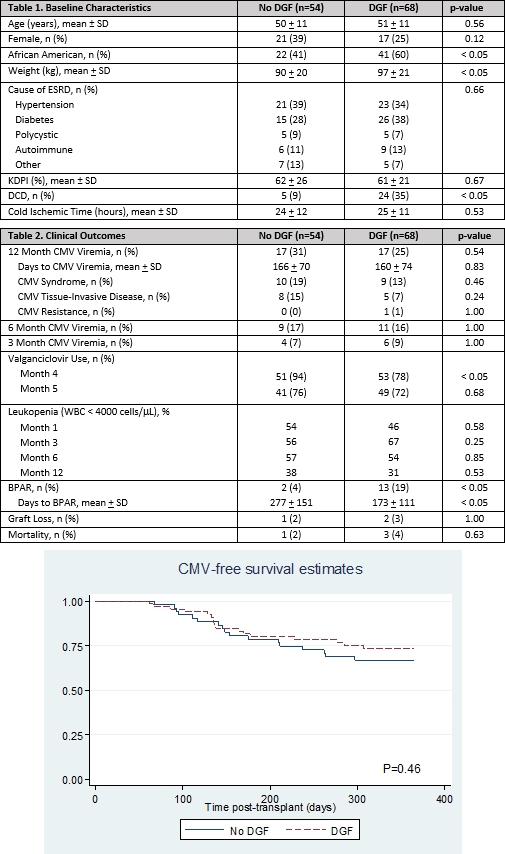
Results: A total of 122 patients were included. Differences in baseline characteristics (table 1) were not predictive of CMV viremia by univariate analysis. Maintenance IS was similar at months 1, 3, 6, and 12 posttransplant. CMV viremia by 12 months was similar (31% no DGF vs 25% DGF, p=0.54) (table 2) and independent of DGF duration (median 14 days (8-23)). The mean time to CMV viremia was less than 6 months in both groups (table 2, figure 1) with fewer DGF patients on prophylaxis at 4 months posttransplant. Greater than 50% of patients in both arms experienced leukopenia from months 3 to 6, but few patients experienced neutropenia (ANC < 1000 cells/mcL). There were significantly more BPAR episodes in patients with DGF, however BPAR never preceded CMV. One year patient and graft survival did not differ between groups.
Conclusion: These data suggest that valganciclovir 450 mg twice weekly is an effective strategy to prevent CMV in kidney transplant recipients who experience DGF. Although leukopenia from alemtuzumab induction may coincide with the 6 month period of valganciclovir prophylaxis, efforts should be made to continue valganciclovir for the full duration of prophylaxis.
CITATION INFORMATION: Freedman S., Ravichandran B., Masters B., Heil E., Saharia K., Bromberg J., Haririan A., Sparkes T. Cytomegalovirus Prophylaxis Following Alemtuzumab Induction in High Risk Renal Transplant Recipients Experiencing Delayed Graft Function Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Freedman S, Ravichandran B, Masters B, Heil E, Saharia K, Bromberg J, Haririan A, Sparkes T. Cytomegalovirus Prophylaxis Following Alemtuzumab Induction in High Risk Renal Transplant Recipients Experiencing Delayed Graft Function [abstract]. https://atcmeetingabstracts.com/abstract/cytomegalovirus-prophylaxis-following-alemtuzumab-induction-in-high-risk-renal-transplant-recipients-experiencing-delayed-graft-function/. Accessed February 18, 2026.« Back to 2018 American Transplant Congress