Covid19 in Pediatric Kidney Transplant Recipients: Incidence, Outcomes, and Response to Vaccine
Nephrology, Children's National Med. Ctr., Washington, DC
Meeting: 2022 American Transplant Congress
Abstract number: 819
Keywords: COVID-19, Kidney, Outcome, Vaccination
Topic: Clinical Science » Kidney » 43 - Kidney: Pediatrics
Session Information
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
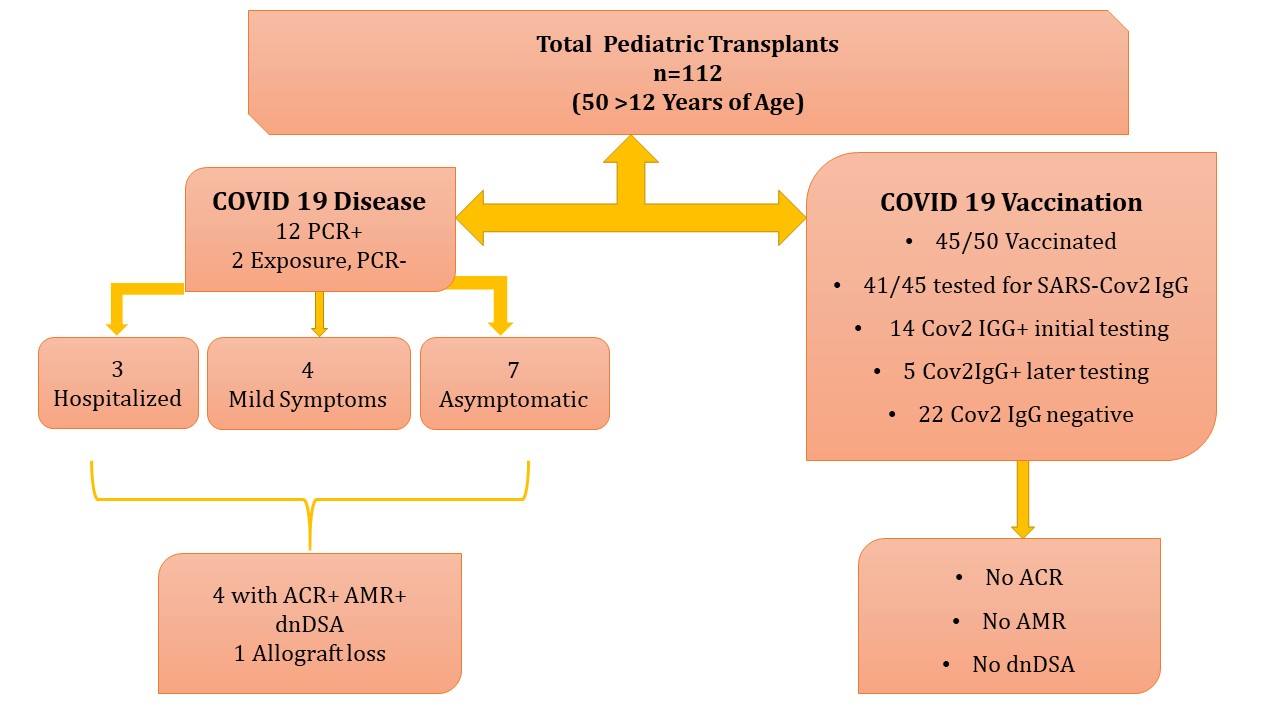
*Purpose: At the onset of pandemic, no information was available on response of pediatric kidney transplant (PKTX) recipients to COVID19. When COVID19 vaccines became available, response of immunosuppressed children to the vaccines was also not known. Here we present information on response to COVID19 and its vaccine in PKTX recipients.
*Methods: Since March 2020, we systematically collected information on the incidence of COVID19 exposure, COVID19 disease and complications, effects on allograft function in all PKTX followed at Children’s National Hospital (CNH). Data on clinical and SARS Cov2 IgG (measured 3-4 weeks after 2nd dose and repeated 1-2 months later if negative) in vaccine recipients was collected.
*Results: Of 112 PKTX (age 2-21 years) at CNH, 12 (10.7%) had COVID19 RNA detected in nasopharyngeal (NP) swabs, 2 children tested negative post exposure. Of 12 with COVID19, 3 were hospitalized with fever and cough but did not require intensive care, 4 had mild symptoms (cough, loss of smell and body aches), and 5 were asymptomatic. Four of 12 with COVID19 developed dnDSA and biopsy proven mixed ACR and AMR; 3 of these had non-adherence to immunosuppression. One child lost allograft despite treatment; 3 responded partially (figure). Of 50 (>12 years) children eligible for vaccine, 45 (90%,15 LR, 30 DD) received it 4.2+ 3.6 (0.16-14.5) years after transplant. Pfizer vaccine was given to 39, Moderna to 5 and Johnson and Johnson to one. Eight of 45 (17.8%) had mild side effects to the vaccine including sore arm, tiredness, and muscle aches. None required hospitalization nor developed dnDSA, ACR or AMR. Of 41 vaccine recipients tested for SARS Cov2 IgG, 14 (34%) were positive at initial testing, 5 (12.8%) had antibody detected on repeat testing, 22 (53.6%) have remained negative (figure). Of 14 who tested positive upon initial testing, 8 were diagnosed with COVID19 and 2 had COVID19 exposure before receiving the vaccine. No vaccine recipients have been diagnosed with COVID19.
*Conclusions: A minority of pediatric kidney transplant recipients developed COVID19 during the pandemic, most had mild symptoms. A small number of these children developed allograft rejection in combination with non-adherence to immunosuppression. Most children tolerated COVID vaccination without any significant side effects or development of ACR, AMR or dnDSA. Nearly 1/2 of PKTX recipients did not respond to vaccine after completing the original series and may benefit from a booster. It is possible that prior COVID19 disease or exposure may have boosted response to the vaccine in some children.
To cite this abstract in AMA style:
Moudgil A, Sgambat K, Shi Y, Meyers M, Petyak C, Midgley L. Covid19 in Pediatric Kidney Transplant Recipients: Incidence, Outcomes, and Response to Vaccine [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/covid19-in-pediatric-kidney-transplant-recipients-incidence-outcomes-and-response-to-vaccine/. Accessed February 26, 2026.« Back to 2022 American Transplant Congress