Conversion from Tacrolimus to Belatacept in Pancreas Transplant Alone Recipients with Chronic Kidney Disease.
Medicine/Nephrology & Surgery/Transplant, Indiana University, Indianapolis.
Meeting: 2016 American Transplant Congress
Abstract number: 486
Keywords: Calcineurin, Kidney, Pancreas transplantation, Renal dysfunction
Session Information
Session Name: Concurrent Session: Clinical Pancreas Transplantation 2
Session Type: Concurrent Session
Date: Tuesday, June 14, 2016
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:18pm-5:30pm
Presentation Time: 5:18pm-5:30pm
Location: Room 210
Background: CNI toxicity is a well known risk factor for chronic kidney disease and ESRD in organ transplants. We report our experience in converting Pancreas Transplant Alone (PTA) recipients from tacrolimus to Belatacept in order to avoid further worsening of kidney function.
Methods: Six (mean age = 52.8+/-7, 5 Females, 5 Caucasians) patients with PAT initially maintained on tacrolimus, sirolimus and mycophenolate with biopsy proven native chronic kidney atrophy & fibrosis consistent with CNI toxicity were enrolled in a 1 year prospective intention to treat IRB approved study. Tacrolimus was weaned off over 60 days. Patients were maintained on a steroid free regimen of Belatacept, Sirolimus (level 3-6ng/ml) and Mycophenolate.
Results: Median time from PAT to conversion was 5.9 years(range 2.5-9.5). Pre-conversion the mean eGFR was 28.9+/-9, that modestly improved over 12 months to an eGFR of 32.7+/-11. 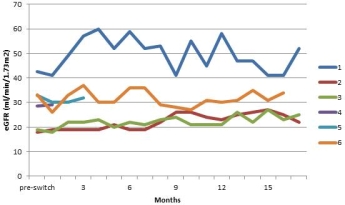 One patient could not tolerate the oral immunosuppression regimen and another patient was non compliant and hence did not complete the study. The remaining 4 cases completed 12 months, of which only 1 case experienced elevation of Lipase requiring steroid therapy with subsequent successful response and continued on the same regimen with Belatacept. Serum glucose, C-peptide and Hemoglobin A1c levels remained unchanged over the study period. There was no incidence of BK, CMV, EBV, PTLD or Donor Specific Antibody (DSA) noted during prospective monitoring. No other new clinically significant event was noted with the use of this regimen over these 12 months. These 4 cases now have each completed a mean follow up of 17 months without any other new significant events with stable renal function and proteinuria.
One patient could not tolerate the oral immunosuppression regimen and another patient was non compliant and hence did not complete the study. The remaining 4 cases completed 12 months, of which only 1 case experienced elevation of Lipase requiring steroid therapy with subsequent successful response and continued on the same regimen with Belatacept. Serum glucose, C-peptide and Hemoglobin A1c levels remained unchanged over the study period. There was no incidence of BK, CMV, EBV, PTLD or Donor Specific Antibody (DSA) noted during prospective monitoring. No other new clinically significant event was noted with the use of this regimen over these 12 months. These 4 cases now have each completed a mean follow up of 17 months without any other new significant events with stable renal function and proteinuria.
Conclusions: Chronic kidney disease progression due to CNI toxicity can likely be stabilized by converting to Belatacept in PTA recipients. Larger and longer term studies are needed to ensure the safety of this approach in PTA recipients in order to preserve kidney function.
CITATION INFORMATION: Sharfuddin A, Chen J, Yaqub M, Taber T, Powelson J, Mishler D, Goble M, Fridell J, Adebiyi O, Mujtaba M. Conversion from Tacrolimus to Belatacept in Pancreas Transplant Alone Recipients with Chronic Kidney Disease. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Sharfuddin A, Chen J, Yaqub M, Taber T, Powelson J, Mishler D, Goble M, Fridell J, Adebiyi O, Mujtaba M. Conversion from Tacrolimus to Belatacept in Pancreas Transplant Alone Recipients with Chronic Kidney Disease. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/conversion-from-tacrolimus-to-belatacept-in-pancreas-transplant-alone-recipients-with-chronic-kidney-disease/. Accessed March 2, 2026.« Back to 2016 American Transplant Congress
