Conversion from Tacrolimus Immediate Release to Tacrolimus Extended Release (Envarsus XR®): A Real World Cohort Highlighting Dosing Variability among Patients
1Department of Pharmacy, Yale New Haven Hospital, New Haven, CT, 2Yale University School of Medicine, New Haven, CT, 3University of Connecticut School of Pharmacy, Storrs, CT
Meeting: 2019 American Transplant Congress
Abstract number: A242
Keywords: Calcineurin, Immunosuppression, Kidney transplantation
Session Information
Session Name: Poster Session A: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Session
Date: Saturday, June 1, 2019
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Hall C & D
*Purpose: Tacrolimus extended release (TacER) (Envarsus XR®) has become an attractive alternative to tacrolimus immediate release (TacIR) due to its once daily dosing scheme, favorable kinetic profile and potential for reduced adverse effects. While the TacER package insert recommends an 80% conversion from the total daily TacIR dose, necessary conversions between 70-85% were noted in the phase II and III studies for TacER. The objective of this study was to determine the percent conversion from TacIR to TacER in a real world cohort.
*Methods: A retrospective chart review of patients transplanted between 01/2017 and 09/2018 was conducted. Adult kidney transplant recipients > 1 month from transplant who were converted from TacIR to TacER and obtained two consecutive therapeutic levels were included. The primary endpoint was percent conversion of total daily dose from TacIR to TacER. Secondary outcomes included number of necessary dose adjustments, time from conversion to two therapeutic levels, and percent conversion by race/ethnicity.
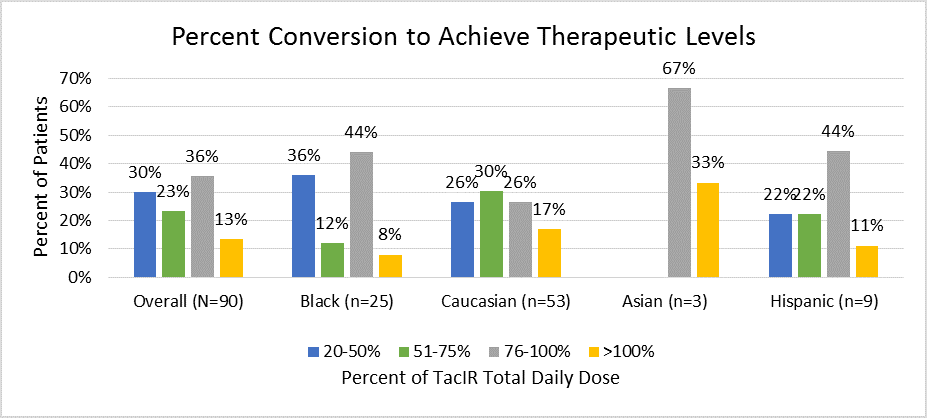
*Results: Two hundred and sixteen patients were transplanted during the study period, 118 were approached to convert from TacIR to TacER and 90 patients were converted and achieved two consecutive therapeutic levels. Average age was 52.3 years and 56% of patients were male. Fifty-nine percent of patients were Caucasian, 28% were Black, 10% were Hispanic and 3% were Asian. The overall average percent conversion was 82.3±39.6. However there was wide variability as seen in figure below. An average of 1.38±1.5 dose adjustments were required to reach two consecutive therapeutic levels over a median of 53 days.
*Conclusions: While the average percent conversion from TacIR to TacER was consistent with the package insert, there was wide variability with 30% of patients requiring <50% and 13% of patients requiring >100% conversion. Our results highlight the need for close monitoring and adjustment for patients converted to TacER and further investigation of factors affecting dose conversion.
To cite this abstract in AMA style:
Belfield KD, Malhotra D, Waleed M, Cohen EA. Conversion from Tacrolimus Immediate Release to Tacrolimus Extended Release (Envarsus XR®): A Real World Cohort Highlighting Dosing Variability among Patients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/conversion-from-tacrolimus-immediate-release-to-tacrolimus-extended-release-envarsus-xr-a-real-world-cohort-highlighting-dosing-variability-among-patients/. Accessed March 5, 2026.« Back to 2019 American Transplant Congress