Continuous 24-Hour Antithymocyte Globulin (ATG) for Renal Allograft Rejection: Results of a Randomized Controlled Trial
Houston Methodist Hospital, Houston, TX
Meeting: 2021 American Transplant Congress
Abstract number: 891
Keywords: Antilymphocyte antibodies, Immunosuppression, Kidney transplantation
Topic: Clinical Science » Kidney » Kidney: Acute Cellular Rejection
Session Information
Session Name: Kidney: Acute Cellular Rejection
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: For acute renal allograft rejection (AR), ATG is generally administered in the inpatient setting because of the need for intravenous access, patient monitoring, and daily administration. Herein, we report the initial outcomes of a trial evaluating the feasibility of a 24-hour ATG infusion to reduce inpatient length of stay (LOS).
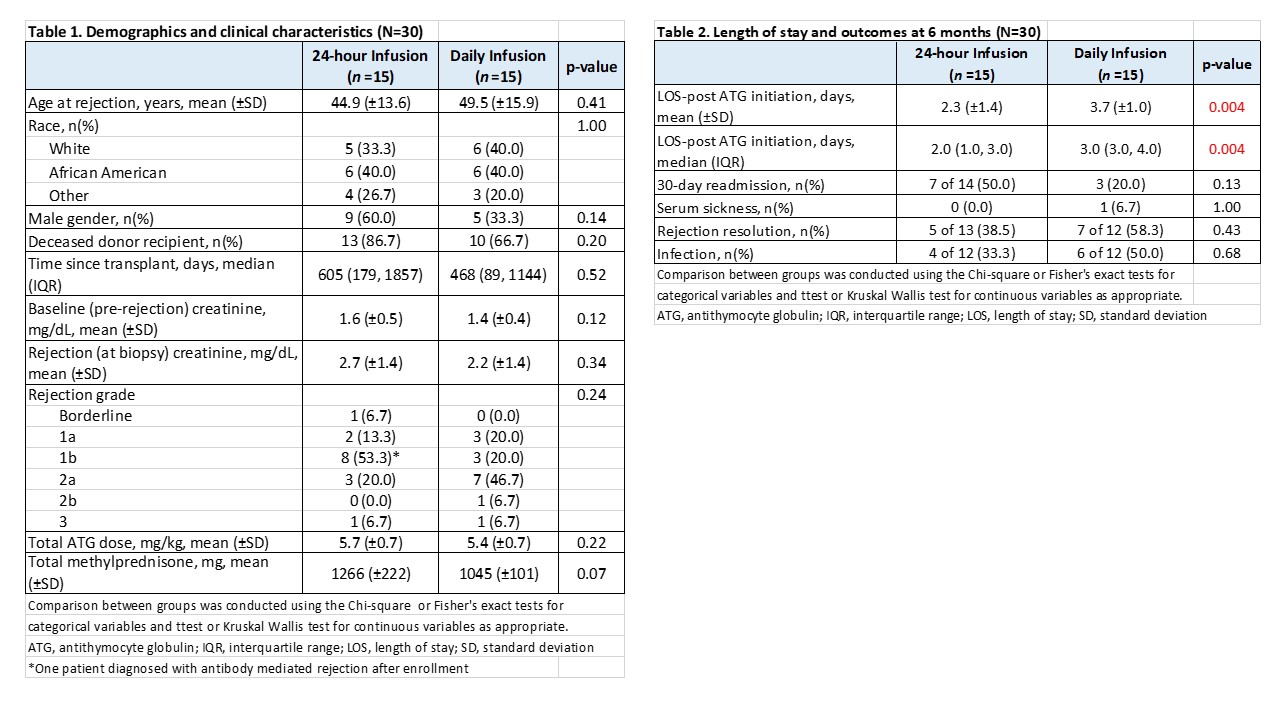
*Methods: Patients requiring ATG (Thymoglobulin®) for treatment of biopsy-proven AR were randomized 1:1 to receive either a 24-hour continuous infusion of ATG 6 mg/kg (24-hour arm) or 4 daily infusions of 1.5 mg/kg, each over 6 hours (Daily arm). Pre-medications were methylprednisolone, diphenhydramine and acetaminophen administered every 6 hours starting prior to the infusion in the 24-hour group and prior to each daily infusion in the Daily group. Patients with concomitant antibody mediated rejection (AMR) were excluded. The primary endpoint of the study was LOS defined as the number of overnight stays post-ATG initiation. Secondary endpoints included side effects occurring during the infusions, rejection resolution (defined as absence of AR on repeat biopsy 2-3 weeks post-treatment), 30-day readmissions, infections, renal function, and hematologic laboratory values through 6 months post-treatment.
*Results: To date, 30/30 patients have been randomized, all have reached the primary endpoint analysis and 24/30 have completed 6 months of follow-up. One 24-hour patient was diagnosed with AMR after enrollment and required an extended LOS for treatment but was included in the current analysis. Baseline characteristics were similar between groups (Table 1). 24-hour ATG was discontinued after 75% of the infusion in one patient due to nausea and anxiety, and temporarily held in another patient due to chills and fatigue. All other patients tolerated the infusion well. Mean infusion time for the 24-hour group was 25±5.2 hours. Mean LOS following ATG initiation was 2.3±1.4 days for the 24-hour group vs. 3.7±1.0 days for the Daily group (p=0.004, Table 2). No differences were seen in vital signs or adverse effects between 24-hour and Daily infusion groups, nor were there significant differences in hematologic parameters, renal function and other post-treatment outcomes through 6 months (Table 2). Graft loss occurred a week after ATG initiation in one 24-hour patient who presented with acute and chronic rejection 7.7 years post-transplant.
*Conclusions: Continuous infusion ATG over 24 hours for AR is feasible and significantly reduces inpatient LOS. Complete follow-up on the remaining patients is ongoing.
To cite this abstract in AMA style:
Patel SJ, Rogers A, Kuten S, Knight R, Graviss E, Nguyen D, Ellimuttil T, Agboli I, Gaber L, Gaber A. Continuous 24-Hour Antithymocyte Globulin (ATG) for Renal Allograft Rejection: Results of a Randomized Controlled Trial [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/continuous-24-hour-antithymocyte-globulin-atg-for-renal-allograft-rejection-results-of-a-randomized-controlled-trial/. Accessed February 20, 2026.« Back to 2021 American Transplant Congress