Compliance with OPTN Living Kidney Donor Follow-Up Policy Remains Low
1University of North Carolina, Chapel Hill, NC, 2Johns Hopkins, Baltimore, MD
Meeting: 2020 American Transplant Congress
Abstract number: 389
Keywords: Living donor
Session Information
Session Name: Quality Assurance Process Improvement & Regulatory Issues II
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:27pm-4:39pm
Presentation Time: 4:27pm-4:39pm
Location: Virtual
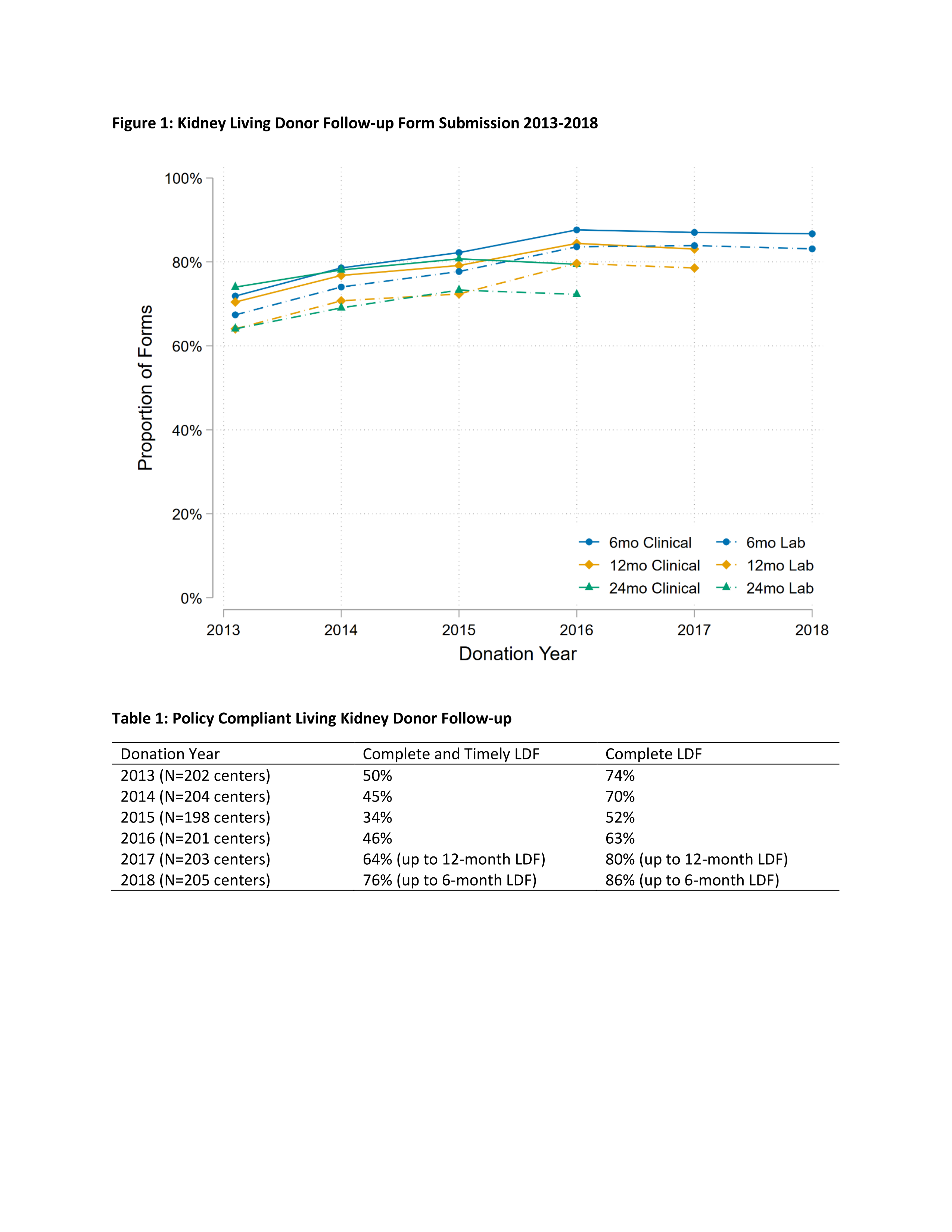
*Purpose: OPTN implemented a policy in 2013 that required all centers to perform 6-, 12-, and 24-month living donor follow-up (LDF). Early compliance with this policy was low, with less than 50% of centers compliant with complete and timely follow-up for living kidney donors. A policy change to be implemented in 2020 will expand the allowable reporting window. In order to assess whether follow-up has improved and the potential impact of the 2020 LDF policy change, we measured policy compliant LDF for living kidney donors through 2018.
*Methods: Using SRTR data on 33,861 living kidney donors between 02/2013 (policy start) and 12/2018, we studied LDF compliance as defined by OPTN, i.e. meeting thresholds for the proportion of complete LDF clinical and laboratory data submitted in a timely manner (60 days before or after the expected visit date). We also assessed complete follow-up ignoring the timeliness of submission. Donors transplanted in 2013-2016 were eligible for full 24-month LDF, 2017 donors were eligible for 6- and 12-month LDF, and 2018 donors were eligible for 6-month LDF.
*Results: Only 32 out of 219 (15%) of centers remained fully compliant with follow-up since the policy began in 2013. Ignoring the current 120-day reporting window, 71 (32%) centers met LDF completion thresholds since 2013. The total proportion of complete and timely 6-month LDF forms has plateaued since 2016 (Figure 1). No more than 50% of centers met complete and timely 6-, 12-, and 24-month follow-up for a given annual cohort of donors (Table 1).
*Conclusions: Compliance with the living kidney donor follow-up policy has not improved since the last national study. For a given annual cohort of donors, no more than 50% of centers met the OPTN requirements for 6-, 12-, and 24-month follow-up. Expanding the data submission window might improve compliance rates, but donor- or center-level interventions will be necessary to significantly improve follow-up of living kidney donors.
To cite this abstract in AMA style:
Thomas A, Shaffer A, Segev D, Henderson M. Compliance with OPTN Living Kidney Donor Follow-Up Policy Remains Low [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/compliance-with-optn-living-kidney-donor-follow-up-policy-remains-low/. Accessed July 18, 2025.« Back to 2020 American Transplant Congress