CNI-Free Therapy with Iscalimab (anti-CD40 mAb) Preserves Allograft Histology Compared to Standard of Care after Kidney Transplantation
1Univ of Michigan, Ann Arbor, MI, 2Hospital do Rim, São Paulo, Brazil, 3Univ. Hamburg, Hamburg, Germany, 4Univ Essen, Essen, Germany, 5Erasmus MC, Rotterdam, Netherlands, 6UMC Groningen, Gröningen, Netherlands, 7Univ of Kansas, Kansas City, KS, 8Saint Barnabas MC, Livingston, NJ, 9East Carolina Univ, Greenville, NC, 10Univ of Cincinnati, Cincinnati, OH, 11Henry Ford Hosp, Detroit, MI, 12Charité Univ, Berlin, Germany, 13Univ Heidelberg, Heidelberg, Germany, 14Univ of Colorado SM, Denver, CO, 15Univ MC, Utrecht, Netherlands, 16Novartis, Basel, Switzerland
Meeting: 2019 American Transplant Congress
Abstract number: 632
Keywords: Biopsy, Co-stimulation, Kidney transplantation
Session Information
Session Name: Concurrent Session: Late Breaking Oral Abstract
Session Type: Concurrent Session
Date: Tuesday, June 4, 2019
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:42pm-5:54pm
Presentation Time: 5:42pm-5:54pm
Location: Room 206
*Purpose: To assess if calcineurin (CNI)-free therapy with iscalimab preserves the quality of transplanted kidney grafts.
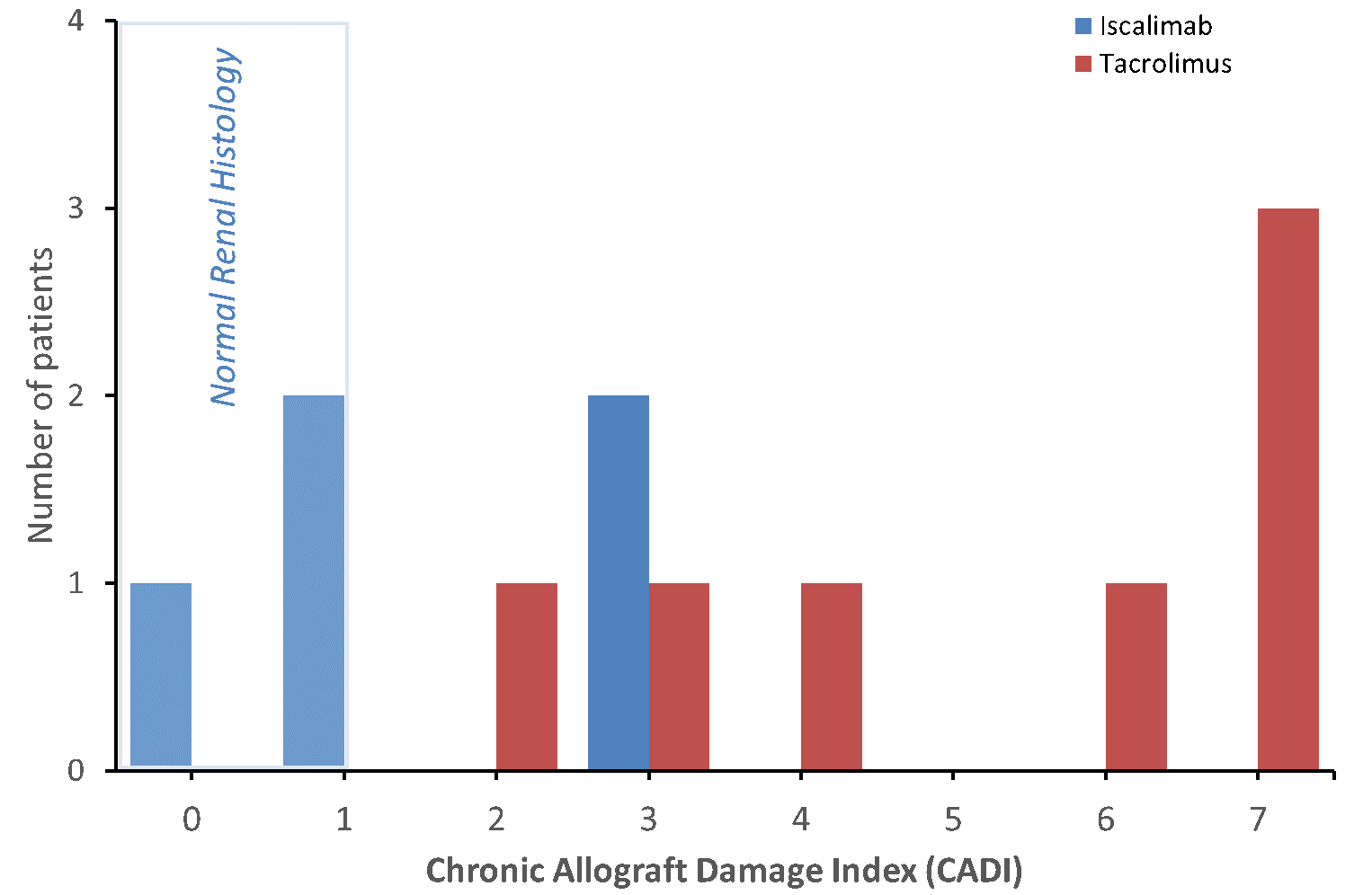
*Methods: Iscalimab (CFZ533) is a new, fully human, Fc-silenced, non-depleting, IgG1 mAb preventing CD40 pathway signaling and activation of CD40+ cell types. In a recent multicenter RCT (NCT02217410), iscalimab therapy showed improved renal function, reduced risk for new onset diabetes and similar safety compared to tacrolimus. Allograft biopsies, although not required as per study protocol, were performed on a subset of study patients. At one site patients were offered extended therapy and a separate protocol allowed for biopsies. A pathologist, blinded to therapy, reviewed and scored all biopsy slides using the established Banff criteria and calculated the chronic allograft damage index (CADI). A CADI of 1 or less was considered as ‘normal renal histology’. Two patients were excluded from the analysis, since they switched therapy after only 2 months.
*Results: Figure 1 shows the CADI for 5 patients treated with iscalimab and 7 patients or matched controls for up to 24 months. Three of five patients (60%) on iscalimab had ‘normal renal histology’ versus none of seven on tacrolimus, p<0.01. The average CADI at final biopsy was 1.6 ±0.6 for iscalimab and 5.1 ±0.8 for tacrolimus, p<0.01. One patient on iscalimab, with a CADI score 3, had significant BK viremia, 20K copies/ml.
*Conclusions: Compared to current standard-of-care, iscalimab appears to be associated with lower CADI scores, with close to normal histology maintained in a high proportion of allografts. These findings, albeit in a limited number of patients, confirms observations in nonhuman primates, and are to be confirmed in the ongoing Phase 2b trial (Cirrus I, NCT03663335). We conclude that iscalimab has the potential to improve long-term outcomes.
To cite this abstract in AMA style:
Farkash E, Naik A, Tedesco-Silva H, Nashan B, Witzke O, Hoogen Mvanden, Berger S, Cibrik DM, Mulgaonkar S, Leeser DB, Alloway R, Patel A, Pratchke J, Sommerer C, Wiseman A, Zuilen AVan, Laessing U, Rush J, Haraldsson B. CNI-Free Therapy with Iscalimab (anti-CD40 mAb) Preserves Allograft Histology Compared to Standard of Care after Kidney Transplantation [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/cni-free-therapy-with-iscalimab-anti-cd40-mab-preserves-allograft-histology-compared-to-standard-of-care-after-kidney-transplantation/. Accessed March 9, 2026.« Back to 2019 American Transplant Congress