Clostridium difficile Infection in Patients with a Continuous Flow, Durable Left Ventricular Assist Device.
NewYork-Presbyterian Hospital, Columbia University, New York.
Meeting: 2016 American Transplant Congress
Abstract number: B160
Keywords: Heart assist devices, Infection, Risk factors, Ventricular assist devices
Session Information
Session Name: Poster Session B: Hearts and VADs in Depth - The Force Awakens
Session Type: Poster Session
Date: Sunday, June 12, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Clostridium difficile infection (CDI) increases morbidity and mortality in hospitalized patients. Patients with a ventricular assist device (VAD) are exposed to known risk factors for CDI including antimicrobials, acid suppressive therapy, frequent hospitalizations, and feeding tubes. We sought to evaluate risk factors for CDI in this patient population.
Methods: Retrospective chart review of adult patients who received a VAD from January 2010 through December 2013. Patients with a positive C. difficile polymerase chain reaction (PCR) at least 24 hours after VAD implantation were cases and those with a negative PCR were controls. The primary outcome was to assess risk factors for CDI after VAD placement. Secondary outcomes included treatment of CDI, and CDI recurrence.
Results: Of the 185 patients who received a VAD, 48 had a positive C. difficile PCR. Baseline characteristics were similar between cases and controls. The majority of patients were middle-aged white males with a Heartmate II. Those with CDI were more likely to have received a histamine-2 receptor antagonist, antimalarial agent, or cephalosporin within 3 months preceding CDI diagnosis. Cases also had higher cumulative antimicrobial exposure (25 vs 13 days p=0.004). The majority of patients who developed CDI were treated with monotherapy and 42% required therapy escalation. Approximately one-third of cases developed CDI recurrence within 8 weeks of completing initial treatment. 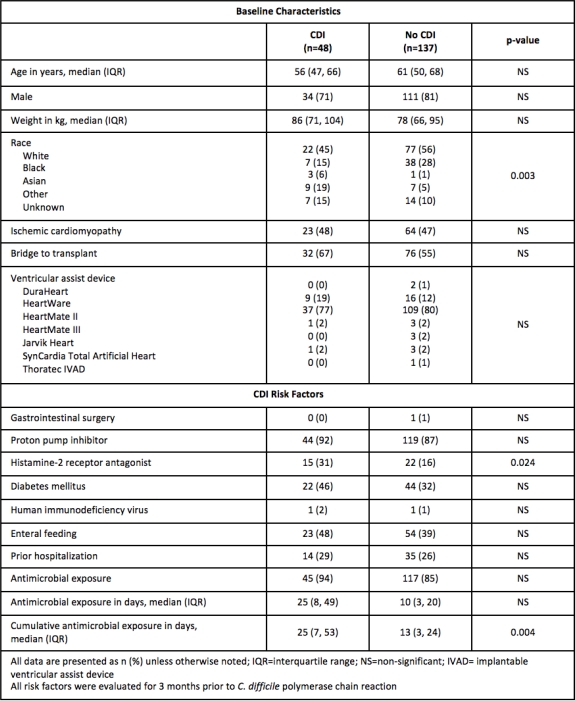
Conclusion: Careful antimicrobial selection and efforts to decrease antimicrobial and acid suppressive therapy exposure in VAD patients is prudent. Clinicians should have heightened awareness of CDI recurrence with diligent monitoring following treatment.
CITATION INFORMATION: Glick Frasiolas J, Li I, Kim H, Tsapepas D. Clostridium difficile Infection in Patients with a Continuous Flow, Durable Left Ventricular Assist Device. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Frasiolas JGlick, Li I, Kim H, Tsapepas D. Clostridium difficile Infection in Patients with a Continuous Flow, Durable Left Ventricular Assist Device. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/clostridium-difficile-infection-in-patients-with-a-continuous-flow-durable-left-ventricular-assist-device/. Accessed March 9, 2026.« Back to 2016 American Transplant Congress
