Clinically Relevant Wound Events With Everolimus-Based Immunosuppressive Regimen: Post-Hoc Analysis from a Randomized Heart Transplant Study
1For the A2309 Study Group, Vienna, Austria
2Novartis, Basel, Switzerland.
Meeting: 2015 American Transplant Congress
Abstract number: 75
Keywords: Adverse effects, Heart transplant patients, Immunosuppression
Session Information
Session Name: Concurrent Session: "The Pit and the Pendulum": VADs, Dual Organs and Other Matters of the Heart
Session Type: Concurrent Session
Date: Sunday, May 3, 2015
Session Time: 2:15pm-3:45pm
 Presentation Time: 3:27pm-3:39pm
Presentation Time: 3:27pm-3:39pm
Location: Room 119-B
Purpose: In the A2310 (NCT00300274) heart transplant (HTx) study with everolimus (EVR) vs mycophenolate mofetil (MMF), information on a broad spectrum of potential wound healing events (WHE), spanning from incision site pain to mediastinitis, were collected prospectively. WHE occurred in 1/3 of patients in all arms with no significantly increased risk for patients on EVR vs MMF. In a post-hoc analysis, clinically relevant WHE, defined by surgical experts, was evaluated to assess a potential increased risk with EVR for critical WHE. Methods: This 24 month (M), open-label, multicenter study randomized 721 HTx recipients to EVR1.5mg (C0 3-8ng/mL; N=282) or EVR3mg (C0 6-12ng/mL; N=168)+reduced cyclosporine (rCsA) or MMF3g (N = 271)+standard (s) CsA; with steroids±induction. Enrollment into the EVR3mg arm was stopped early due to higher mortality. Detailed data were collected on location (sternal/non-sternal), symptoms, degree, diagnosis and intervention on WHE using special case report forms and results were published. Subsequently, the list of reported WHE was reviewed by the surgical experts and those events not considered as clinically relevant were excluded for this post-hoc analysis. WHE reported as serious adverse event, mediastinitis, osteomyelitis, or events requiring surgical debridement, vacuum assisted closure (VAC) or sternal rewiring were considered as critical. Results: Overall, 23.7%, 24.6% and 20.5% of patients experienced at least one clinically relevant WHE in EVR1.5mg, 3mg and MMF arms, respectively. Incidence of sternal and non-sternal WHE was similar across all the arms. Incidence of critical WHE was numerically higher with EVR (1.5mg: 10.0% and 3mg: 15.0%) vs MMF (9.0%) arms, but with overall low incidences for mediastinitis, and wound dehiscence. The patients requiring surgical debridement, rewiring or VAC for sternal WHE between EVR1.5mg and MMF arms was not significantly different (Table). Conclusions: Patients treated with EVR1.5mg up to 12M experienced a comparable incidence of clinically relevant WHE vs MMF. Higher doses of EVR may increase the incidence of WHE.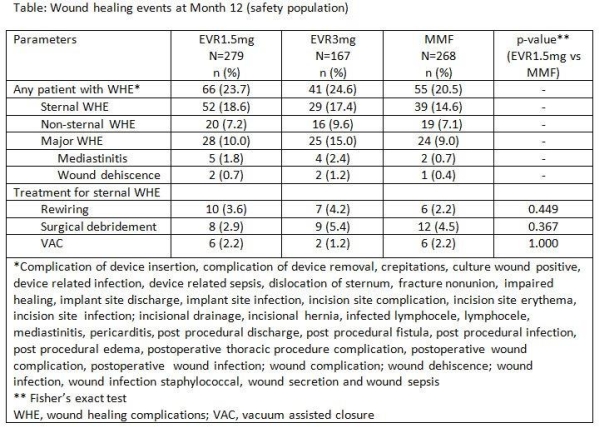
To cite this abstract in AMA style:
Zuckermann A, Rinaldi M, Yonan N, Wang S-S, Dong G, Lopez P. Clinically Relevant Wound Events With Everolimus-Based Immunosuppressive Regimen: Post-Hoc Analysis from a Randomized Heart Transplant Study [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/clinically-relevant-wound-events-with-everolimus-based-immunosuppressive-regimen-post-hoc-analysis-from-a-randomized-heart-transplant-study/. Accessed March 7, 2026.« Back to 2015 American Transplant Congress
