Clinical Outcomes of Solid Organ Transplant (SOT) Patients with Epstein-Barr Virus-Driven (EBV+) Post-Transplant Lymphoproliferative Disease (PTLD) Who Fail Rituximab Plus Chemotherapy: A Multinational, Retrospective Chart Review Study
V. Dharnidharka1, D. Thirumalai2, U. Jaeger3, W. Zhao2, D. Dierickx4, P. Xun2, P. Minga5, A. Sawas6, N. Sadetsky7, P. Chauvet8, E. Sundaram9, A. Barlev7, H. Zimmermann10, R. U. Trappe10
1Washington University School of Medicine/St Louis Children’s Hospital, St Louis, MO, 2Atara Biotherapeutics, Thousand Oaks, CA, 3Medical University of Vienna, Vienna, Austria, 4Universitair Ziekenhuis Leuven, Leuven, Belgium, 5Niguarda Ca’ Granda Hospital, Milan, Italy, 6Herbert Irving Comprehensive Cancer Center, Department of Medicine, CUMC, New York, NY, 7Atara Biotherapeutics, South San Francisco, CA, 8Service des Maladies du Sang, CHU Lille, Lille, France, 9Nashville Biosciences, Nashville, TN, 10DIAKO Bremen, Bremen, Germany
Meeting: 2022 American Transplant Congress
Abstract number: 60
Keywords: Epstein-Barr virus (EBV), Outcome, Post-transplant lymphoproliferative disorder (PTLD), Survival
Topic: Clinical Science » Infection Disease » 28 - PTLD: All Topics
Session Information
Session Name: PTLD and Malignancies
Session Type: Rapid Fire Oral Abstract
Date: Sunday, June 5, 2022
Session Time: 3:30pm-5:00pm
 Presentation Time: 3:40pm-3:50pm
Presentation Time: 3:40pm-3:50pm
Location: Hynes Room 309
*Purpose: EBV+ PTLD patients (pts) following SOT who fail rituximab + chemotherapy (CT) have poor outcomes with limited treatment options. Published data on clinical outcomes of these pts remain limited and not well documented.
*Methods: We conducted a large multinational, multicenter, retrospective chart review study of pts with EBV+ PTLD following allogeneic hematopoietic cell transplantation or SOT who received rituximab or rituximab + CT between Jan 2000‒Dec 2018 and were refractory (failed to achieve CR or PR) or relapsed at any point after such therapy. Data were collected from 29 centers across North America and the European Union. This analysis includes pts with EBV+ PTLD following SOT who were refractory/relapsed to rituximab + CT. Kaplan-Meier method was utilized to estimate the overall survival (OS).
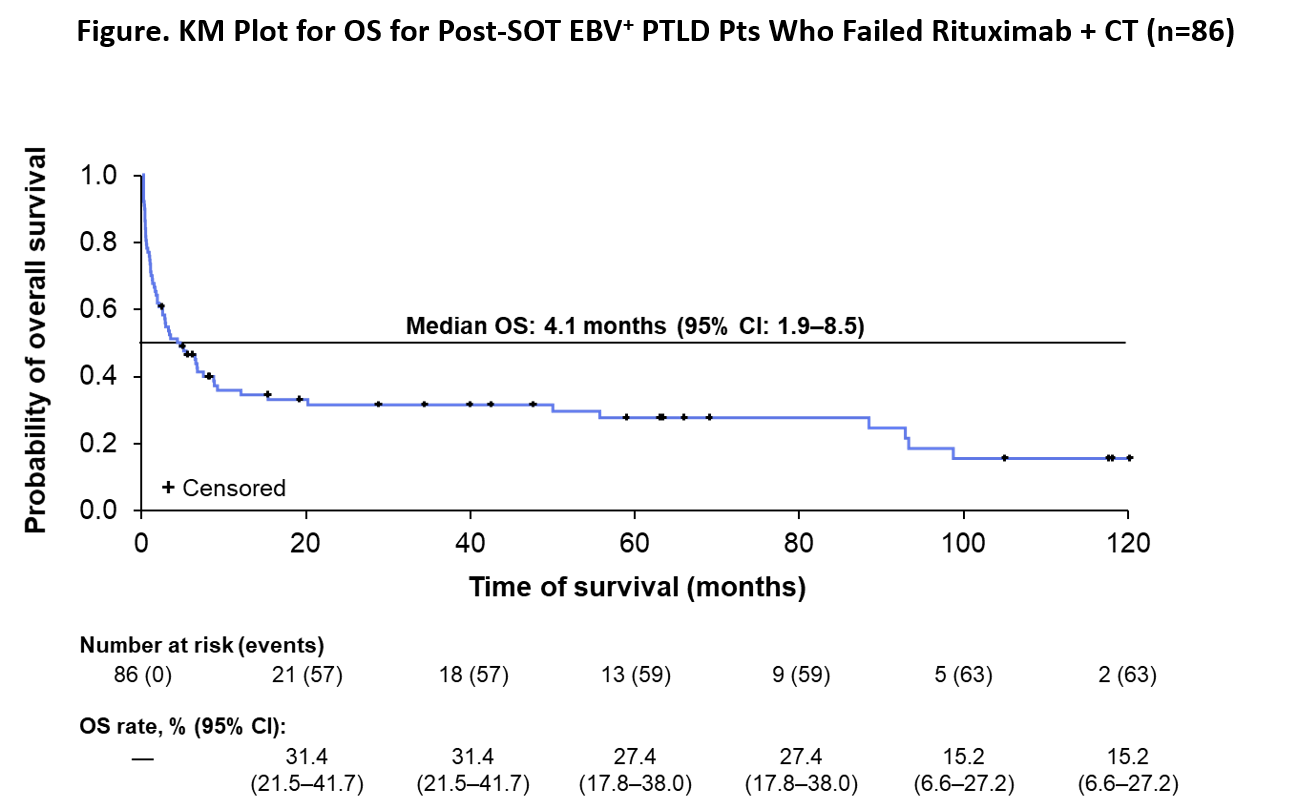
*Results: A total of 86 pts were included in the analysis; 75.6% were refractory while 24.4% relapsed after initial response of CR or PR to rituximab + CT. Median age at PTLD diagnosis was 43 years (range 1‒78) and median time to PTLD onset from transplant was 1.7 years (range 0.1‒27.9). PTLD histological subtypes were 76.7% monomorphic, 20.9% polymorphic, and 2.3% early lesions. Overall, 73.3% of pts died. Causes of death comprised 65.1% PTLD, 15.9% treatment-related mortality, 3.2% organ rejection/failure, 11.1% other causes, and 4.8% unknown. Median OS was 4.1 months (95% CI: 1.9‒8.5) from the date when pts became relapsed/refractory to rituximab + CT (Fig).
*Conclusions: Prognosis for pts with EBV+ PTLD following SOT who fail rituximab + CT remains poor with an estimated median OS of about 4 months and a majority of pts dying from PTLD and related treatment. There remains a significant unmet medical need for effective and well-tolerated therapies.
To cite this abstract in AMA style:
Dharnidharka V, Thirumalai D, Jaeger U, Zhao W, Dierickx D, Xun P, Minga P, Sawas A, Sadetsky N, Chauvet P, Sundaram E, Barlev A, Zimmermann H, Trappe RU. Clinical Outcomes of Solid Organ Transplant (SOT) Patients with Epstein-Barr Virus-Driven (EBV+) Post-Transplant Lymphoproliferative Disease (PTLD) Who Fail Rituximab Plus Chemotherapy: A Multinational, Retrospective Chart Review Study [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/clinical-outcomes-of-solid-organ-transplant-sot-patients-with-epstein-barr-virus-driven-ebv-post-transplant-lymphoproliferative-disease-ptld-who-fail-rituximab-plus-chemotherapy-a-multinationa/. Accessed March 9, 2026.« Back to 2022 American Transplant Congress