Clinical Outcomes of Solid Organ Transplant Recipients Treated with Remdesivir and Convalescent Plasma for Covid-19 at the Largest Transplant Center in the United States
A. Fernandez, S. Anjan, A. Chandorkar, D. de Lima, R. Zamora, L. A. Mendez-Castaner, J. Simkins, J. Camargo, M. Morris, M. Loebe, J. Bauerlein, C. O’Brien, N. Sinha, G. Burke, G. Ciancio, A. Mattiazzi, R. Vianna, L. Abbo, G. Guerra, Y. Natori
Miami Transplant Institute, Jackson Health System, Miami, FL
Meeting: 2021 American Transplant Congress
Abstract number: 21
Keywords: Infection, N/A, Pneumonia, Viral therapy
Topic: Clinical Science » Infectious Disease » All Infections (Excluding Kidney & Viral Hepatitis)
Session Information
Session Name: COVID-19 Session 1
Session Type: Rapid Fire Oral Abstract
Date: Saturday, June 5, 2021
Session Time: 4:30pm-5:30pm
 Presentation Time: 4:40pm-4:45pm
Presentation Time: 4:40pm-4:45pm
Location: Virtual
*Purpose: Solid organ transplant recipients (SOTr) are at high risk for severe disease with SARS-CoV-2. Data on efficacy of potential treatment options and long-term outcomes are lacking. We describe our experience with use of remdesivir and convalescent plasma in SOTr with COVID-19.
*Methods: Single-center, retrospective cohort study of SOTr diagnosed with SARS-CoV-2 infection by PCR from March 1st to September 30th, 2020. Multivariate logistic regression analysis was performed based on univariate analysis to identify the risk factors for higher mortality.
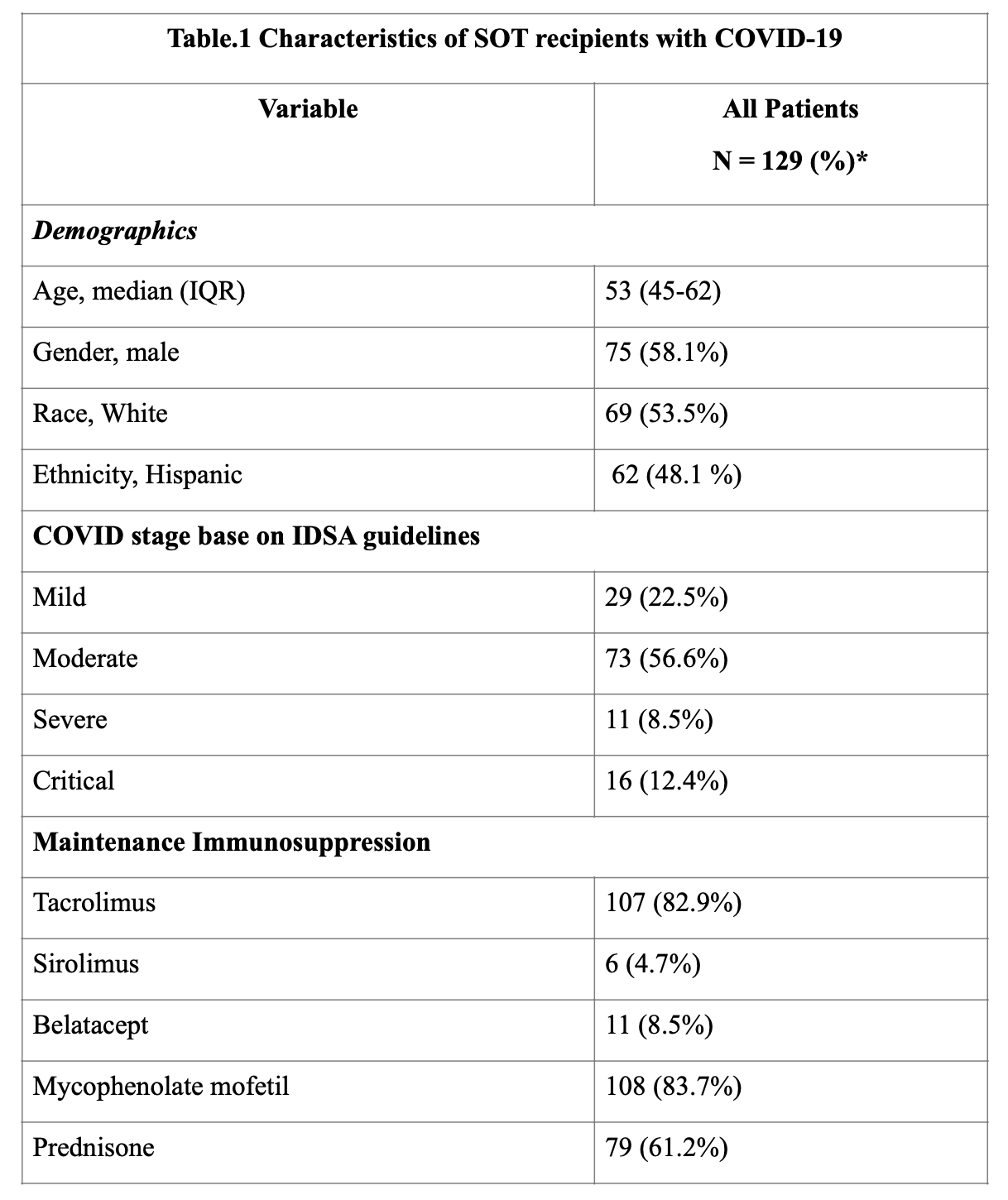
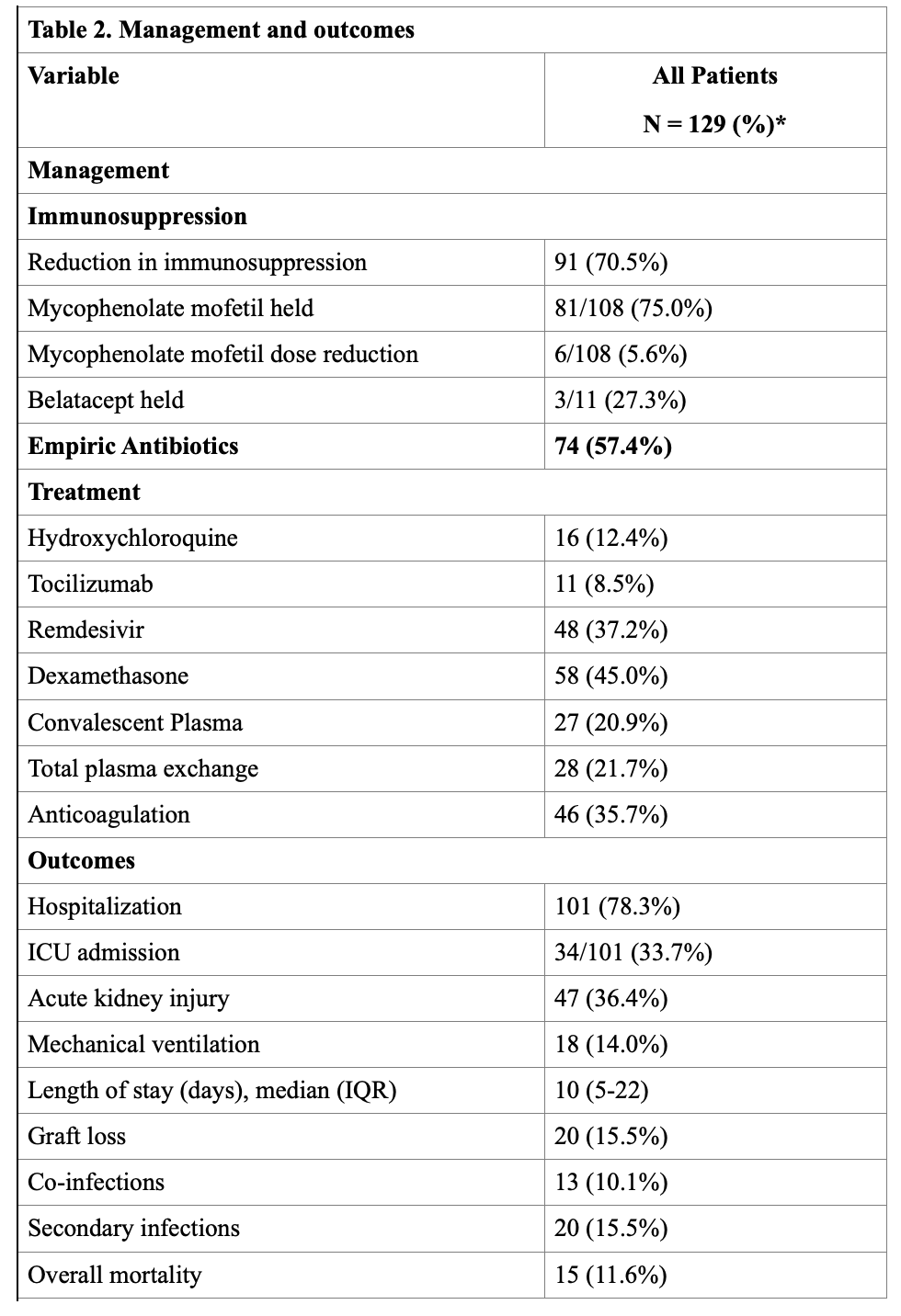
*Results: 129 SOTr were identified (Table. 1). Median time from transplant to diagnosis of infection was 27 (IQR, 8-73) months. 48 (37.2%) and 27 (21%) patients received remdesivir and convalescent plasma, respectively (Table 2). 5/48 (10.4%) patients developed mild transaminitis that did not warrant discontinuation of therapy. No adverse effects were seen with convalescent plasma. Anti-metabolite agents were decreased or stopped in majority of the patients (81%). During follow-up, 12 (9%) patients developed clinically suspected acute rejection. Death, graft loss, and secondary infection occurred in 15 (12%), 20 (16%), and 20 (16%) recipients, respectively. RT-PCR negativity was achieved at a median of 37 (IQR, 25-41) days. Risk factors identified for high mortality were elevated creatinine (p=0.029, Odds ratio[OR] 1.5, 95% Confidence Interval[CI] 1.0- 2.1) and older age (p=0.003, OR 1.1, 95% CI 1.0 – 1.2) at the time of diagnosis.
*Conclusions: SARS-CoV-2 RT-PCR positive SOT recipients in our cohort had favorable outcomes. Use of remdesivir and convalescent plasma was found to be safe. Older age and elevated creatinine at the time of diagnosis were found to be risk factors for higher mortality.
To cite this abstract in AMA style:
Fernandez A, Anjan S, Chandorkar A, Lima Dde, Zamora R, Mendez-Castaner LA, Simkins J, Camargo J, Morris M, Loebe M, Bauerlein J, O’Brien C, Sinha N, Burke G, Ciancio G, Mattiazzi A, Vianna R, Abbo L, Guerra G, Natori Y. Clinical Outcomes of Solid Organ Transplant Recipients Treated with Remdesivir and Convalescent Plasma for Covid-19 at the Largest Transplant Center in the United States [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/clinical-outcomes-of-solid-organ-transplant-recipients-treated-with-remdesivir-and-convalescent-plasma-for-covid-19-at-the-largest-transplant-center-in-the-united-states/. Accessed March 6, 2026.« Back to 2021 American Transplant Congress