Challenges in Examining Transplant Outcomes in Patients with Fontan-Associated Liver Disease
1Children's Mercy Hospital, Kansas City, MO, 2Children's Hospital-Los Angeles, Los Angeles, CA, 3University of Southern California, Los Angeles, CA, 4Mount Sinai Kravis Children's Hospital, New York, NY
Meeting: 2020 American Transplant Congress
Abstract number: C-255
Keywords: Heart failure, Liver cirrhosis, Methodology
Session Information
Session Name: Poster Session C: Non-Organ Specific: Public Policy & Allocation
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: The Fontan procedure has allowed patients born with single ventricle physiology to survive into adulthood. It is projected that the mean age of Fontan patients will be 23 years by 2025, with an estimated global population of 70,000+ Fontan patients. Many patients develop chronic liver disease, termed Fontan-Associated Liver Disease (FALD). Herein, a multidisciplinary group investigated FALD using large clinical databases to better understand the epidemiology of FALD and risk of developing liver failure requiring transplant.
*Methods: A variety of databases were studied, including Cerner Health Facts (more than 500 million patient encounters), Vizient (formerly University HealthSystem Consortium), the Scientific Registry of Transplant Recipients (SRTR), and Society of Thoracic Surgeons (STS) Congenital Heart Surgery Database. Cerner Health Facts: Patient records from 2009-2017 were queried. Patients with single ventricle congenital heart disease were identified using specific diagnostic codes along with CPT codes for the Fontan procedure. These patients were referenced against liver disease ICD codes. SRTR: Potential Fontan patients were identified using the diagnostic code “1207: congenital heart disease with surgery” or a free text diagnosis containing “Fontan” in the heart transplant dataset. These patients were then referenced against the liver transplant waitlist and transplant datasets. The Vizient and STS datasets were not able to identify patients with FALD.
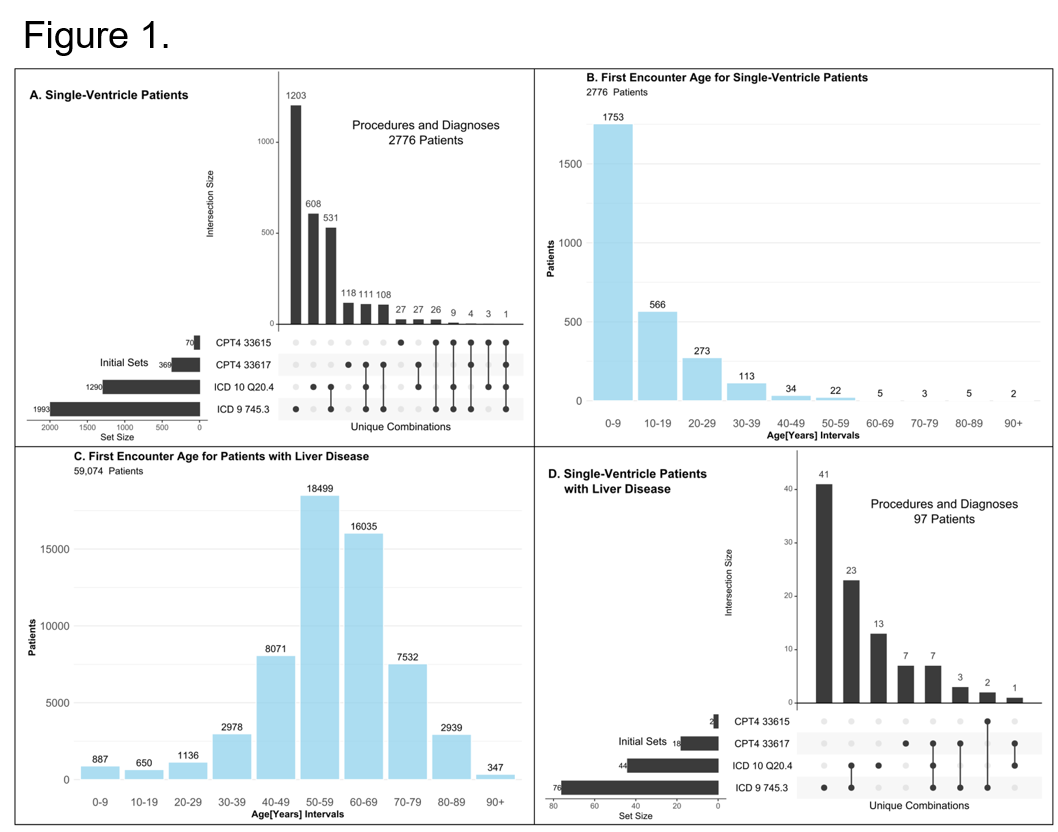
*Results: Analysis of Cerner Health Facts for overlapping diagnostic codes for single ventricle heart disease and chronic liver disease identified a small study population (fewer than 100 patients out of 70 million unique patient records, see Figure 1). Analysis of the SRTR identified nine patients with history of heart transplant for congenital heart disease in the liver transplant waitlist data, with 80% liver waitlist death among adult candidates and only one pediatric patient ultimately receiving a liver transplant.
*Conclusions: The lack of diagnostic codes specific to Fontan or FALD revealed that these patients are not easily identified. Proactive changes including implementing codes to capture these patients and development of large, prospective registries to track FALD will be necessary to understand the role for liver transplant and develop evidence-based treatment guidelines.
To cite this abstract in AMA style:
Fischer RT, Kim MH, Nguyen A, Kumar SR, Bucuvalas J, Glynn EF, Hoffman MA, Emamaullee J. Challenges in Examining Transplant Outcomes in Patients with Fontan-Associated Liver Disease [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/challenges-in-examining-transplant-outcomes-in-patients-with-fontan-associated-liver-disease/. Accessed February 19, 2026.« Back to 2020 American Transplant Congress