CFZ533, a New Anti-CD40 mAB Demonstrates Comparable Efficacy and Better Renal Function versus Tacrolimus in De-Novo CNI-Free Kidney Transplantation
1CFZ533 Study Group, Basel, Switzerland
2Novartis Pharma, Basel, Switzerland.
Meeting: 2018 American Transplant Congress
Abstract number: 400
Keywords: B cells, Co-stimulation, Kidney transplantation
Session Information
Session Time: 8:30am-9:30am
 Presentation Time: 8:45am-9:00am
Presentation Time: 8:45am-9:00am
Location: Room Hall B
Purpose: To assess the potential of CFZ533 as primary immunosuppressant in a calcineurin(CNI)-free regimen in de novo kidney transplant (KTx) patients(pts).
Method: CFZ533 is a new, fully human, Fc-silenced, non-depleting, IgG1 mAb preventing CD40 pathway signaling and activation of CD40+ cell types. NCT02217410 is a 12-month multicenter RCT evaluating efficacy, safety, tolerability, and pharmacokinetics of CFZ533 (CFZ) in combination with mycophenolate mofetil (MMF) and corticosteroids (CS) compared with tacrolimus (TAC), MMF and CS in de novo KTx recipients. All patients received basiliximab induction and corticosteroids as per center practice; a central, blinded pathologist reviewed all allograft biopsies.
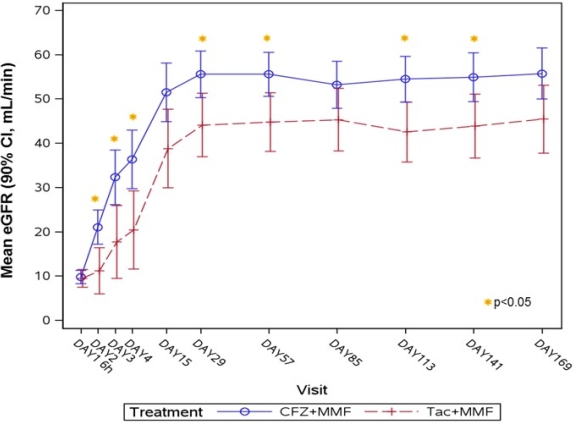
Results: N=51 patients were transplanted and randomized (2:1) to either CFZ (N=33) or TAC (N=18). Twenty-five of 51 pts (49%) received a living donor allograft. After CD40 target saturation, CFZ was dosed IV every 4 weeks. CFZ was well tolerated with no infusion related nor thromboembolic events, and prospective Month 6 interim results demonstrated comparable efficacy on the composite endpoint of treated biopsy proven acute rejection, graft loss, or death (21.2 vs. 22.2%) and better renal function (55.8 vs. 45.5 mL/min) [Fig 1], less serious adverse events (SAE) (47.1 vs. 61.1%) and fewer infection complications (50.0 vs. 77.8%) with no increase of opportunistic infections (viral overall: 26.5 vs. 50.0%; SAE CMV: 2.9 vs. 11.1%; BKV: 15.2 vs. 22.2%), and a lower rate of new-onset diabetes mellitus (14.7 vs. 38.9%) with CFZ vs. TAC, respectively.
Conclusion: CFZ533 may have potential to become an effective CNI-free alternative treatment improving transplant outcomes by preventing graft rejection without nephrotoxic (and other) CNI adverse effects. 12-month final study data will become available in Q1/2018 and will be presented at the 2018 ATC.
Figure 1: Evolution of renal function measured as eGFR (mL/min)
CITATION INFORMATION: Nashan B., Tedesco H., van den Hoogen M., Berger S., Cibrik D., Mulgaonkar S., Leeser D., Alloway R., Patel A., Pratschke J., Sommerer C., Wiseman A., van Zuilen A., Laessing U., Rush J., Haraldsson B., Witzke O. CFZ533, a New Anti-CD40 mAB Demonstrates Comparable Efficacy and Better Renal Function versus Tacrolimus in De-Novo CNI-Free Kidney Transplantation Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Nashan B, Tedesco H, Hoogen Mvanden, Berger S, Cibrik D, Mulgaonkar S, Leeser D, Alloway R, Patel A, Pratschke J, Sommerer C, Wiseman A, Zuilen Avan, Laessing U, Rush J, Haraldsson B, Witzke O. CFZ533, a New Anti-CD40 mAB Demonstrates Comparable Efficacy and Better Renal Function versus Tacrolimus in De-Novo CNI-Free Kidney Transplantation [abstract]. https://atcmeetingabstracts.com/abstract/cfz533-a-new-anti-cd40-mab-demonstrates-comparable-efficacy-and-better-renal-function-versus-tacrolimus-in-de-novo-cni-free-kidney-transplantation/. Accessed February 26, 2026.« Back to 2018 American Transplant Congress