Bortezomib-Based Multi-Modality Protocol for Donor-Specific Antibody Treatment in Lung Transplant Recipients
Duke University Hospital, Durham, NC.
Meeting: 2015 American Transplant Congress
Abstract number: B216
Keywords: Antibodies, Lung transplantation, Rejection
Session Information
Session Name: Poster Session B: Lung- All Topics
Session Type: Poster Session
Date: Sunday, May 3, 2015
Session Time: 5:30pm-6:30pm
 Presentation Time: 5:30pm-6:30pm
Presentation Time: 5:30pm-6:30pm
Location: Exhibit Hall E
Background
Donor specific antibodies (DSAs) after lung transplant have been implicated in the development of chronic lung allograft dysfunction and mortality. The ideal treatment strategy for antibody mediated rejection (AMR) in lung transplant is still unknown. Multiple therapeutic modalities are often used, including plasmapheresis, steroids, intravenous immunoglobulin, and rituximab. In 2010, our institution implemented a novel, aggressive bortezomib-based protocol for the treatment of DSAs in the setting of allograft dysfunction following lung transplantation.
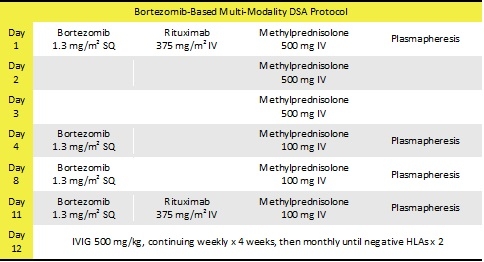
Materials and methods
This study evaluated the programmatic change to initiate this bortezomib-based multi-modality protocol. Lung transplant recipients at least 18 years of age who received at least one dose of bortezomib from the standardized protocol from January 1, 2010 until May 10, 2014 were included. Patients were excluded if they received bortezomib prior to transplant. Primary outcome was clearance of DSAs as defined by mean florescence intensity less than 1000 for all DSAs at 6 months from the first dose of bortezomib.
Results
Twenty-three lung transplant recipients received at least one dose of bortezomib from the protocol, with 61% completing at least four doses. Mean number of doses per patient for bortezomib and rituximab were 3.7 and 2.5, respectively. Six patients did not have DSAs present immediately prior to bortezomib administration but received treatment due to history of DSAs and/or suspected AMR. Of the remaining 17 patients, four patients (23.6%) cleared all DSAs within 6 months. Pulmonary function remained stable (less than 10% decline in FEV1) in 30.4% of patients for 6 months following treatment. Complications included thrombocytopenia (43.5%) and abdominal pain (21.7%). Overall 6 month patient survival was 60.9%.
Conclusion
Bortezomib-based multi-modality protocol results in clearance of DSAs in a low percentage of lung transplant recipients exhibiting AMR. Further study is warranted to determine which patients may benefit from this protocol and whether other clinical outcomes are affected.
To cite this abstract in AMA style:
Vacha M, Hulbert A, Byrns J, Benedetti C, Copeland AFinlen, Gray A, Palmer S, Snyder L. Bortezomib-Based Multi-Modality Protocol for Donor-Specific Antibody Treatment in Lung Transplant Recipients [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/bortezomib-based-multi-modality-protocol-for-donor-specific-antibody-treatment-in-lung-transplant-recipients/. Accessed February 17, 2026.« Back to 2015 American Transplant Congress
