Bisphosphonates for Fracture Prevention in Kidney Transplant Recipients
J. Kahwaji1, S. Yang2, J. Sim3
1Nephrology/Transplant, Kaiser Los Angeles Medical Center, Los Angeles, CA, 2Research and Evaluation, Kaiser-Permanente, Pasadena, CA, 3Nephrology, Kaiser Los Angeles Medical Center, Los Angeles, CA
Meeting: 2022 American Transplant Congress
Abstract number: 764
Keywords: Bone, Kidney transplantation, Metabolic complications, Osteoporosis
Topic: Clinical Science » Kidney » 35 - Kidney: Cardiovascular and Metabolic Complications
Session Information
Session Name: Kidney: Cardiovascular and Metabolic Complications
Session Type: Poster Abstract
Date: Saturday, June 4, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Hall C
*Purpose: Kidney transplant recipients are at increased risk of fracture, however bisphosphonate use has not be shown to lower fracture risk in this population. We aimed to determine if exposure to bisphosphonates was associated with a decreased risk of fractures in kidney transplant recipients.
*Methods: We conducted a retrospective cohort study of Kaiser Permanente Southern California kidney transplant recipients between 2000 and 2019. Patients diagnosed with osteoporosis were included in the study. The exposure of interest was bisphosphonate use after transplant. The primary outcome was non-vertebral fracture. Patients were censored at the time of death, graft failure, or termination of membership. Variables collected included baseline demographics, medications (bisphosphonate, calcitriol, vitamin D, prednisone use), lab tests (electrolytes, Ca, Mg, Phosphorus, PTH, Vitamin D), and comorbidities including DM, hypothyroidism, and rejection history. Chi square was used to evaluate categorical variables and Wilcoxon rank-sum test for continuous variables. To balance covariates and limit indication bias between bisphosphonate users and non-users, inverse probability of treatment weighting was performed to calculate weight for each subject. The obtained weights were applied to Cox proportional model to calculate Hazard ratios (HR).
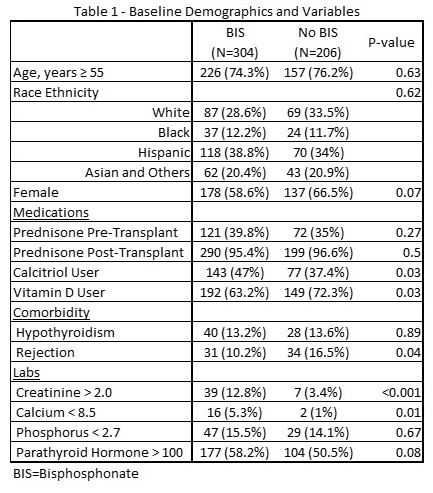
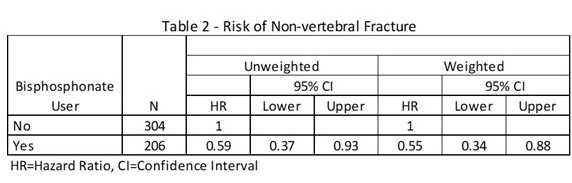
*Results: A total of 510 patients were included in the study totaling 3076 patient-years of follow-up. Baseline characteristics are shown in Table 1. Alendronate was the bisphosphonate of choice 97% of the time. The incidence of non-vertebral fracture was 18.7/1000 person-years for bisphosphonate users and 31.5/1000 person-years for non-users (p=0.03). Compared to non-users, bisphosphonate users had a propensity score weighted HR (95% CI) of 0.55 (0.34-0.88) (Table 2).
*Conclusions: Bisphosphonate use after kidney transplantation was associated with lower risk of non-vertebral fractures. Our findings suggest that screening for osteoporosis and treating with bisphosphonates is beneficial among kidney transplant recipients.
To cite this abstract in AMA style:
Kahwaji J, Yang S, Sim J. Bisphosphonates for Fracture Prevention in Kidney Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/bisphosphonates-for-fracture-prevention-in-kidney-transplant-recipients/. Accessed July 18, 2025.« Back to 2022 American Transplant Congress