Belatacept Maintenance Immunosuppression Use and Outcomes in the Real-World Clinical Setting Using OPTN/UNOS Kidney Transplant Registry
1Duke University Medical Center, Durham, NC
2CTI Consulting, Raleigh, NC.
Meeting: 2018 American Transplant Congress
Abstract number: 120
Keywords: Graft failure, Immunosuppression, Kidney transplantation, Outcome
Session Information
Session Name: Concurrent Session: Kidney Immunosuppression: Co-Stimulation Based Regimens
Session Type: Concurrent Session
Date: Sunday, June 3, 2018
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:06pm-5:18pm
Presentation Time: 5:06pm-5:18pm
Location: Room 6C
Introduction:Belatacept (BEL) was FDA approved for Kidney Transplantation (KTx) June 2011. It has largely been evaluated in two controlled trials (N Engl J Med 2016;374:333-43). We evaluated the real life use and outcomes of BEL maintenance immunosuppression (MIS) using OPTN/UNOS KTx registry data.
Methods: We included adult (≥18) KTx recipients Aug 2011 to Dec 2015 receiving BEL or calcineurin inhibitor (CNI; tacrolimus [TAC] or cyclosporine [CyA]) MIS at discharge. Three groups were identified: Grp1=CNI and No BEL, Grp2=BEL and No CNI, and Grp3=CNI and BEL. Outcomes included all-cause graft failure and death-censored graft failure (DCGF). Treatment selection bias was addressed by fitting a multivariable stratified Cox hazards model adjusting for recipient age, gender, race, history of DM, donor type, donor age, kidney donor risk index, HLA mismatches, cPRA, preemptive KTx and UNOS region. Hazards ratio (HR) and 95% confidence interval (CI) were used. Subgroup analyses were performed in recipients of deceased (DD) and expanded criteria donors (ECD).
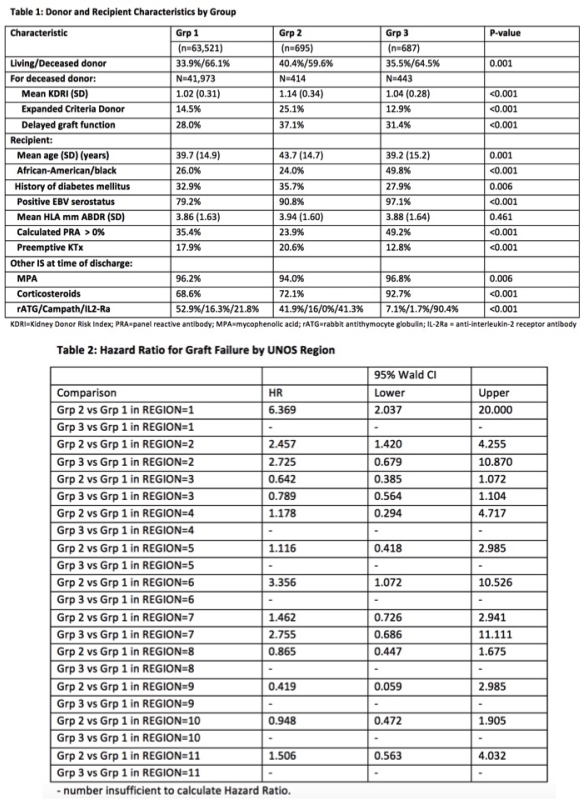
Results: 64,903 patients were included (Grp1: n=63,521 [TAC=98%, CyA=2%], Grp2: n=695 and Grp3: n= 687 [TAC=99%, CyA=1%]. Median follow-up was 25 months. Donor, recipient, and KTx-related characteristics are summarized by Group, Table 1. Of n=1,382 patients who received BEL (Grps2 and 3), >50% were in UNOS region 3. In Grp2 57.9% had lymphocyte depletion vs 45.1% and 8.8% for Grps1 and 3. Kaplan-Meier graft survival at 24-months in Grp1, Grp2 and Grp3 was 92.5%, 91.8% and 93.2%. Adjusted risk of GF and DCGF was not significantly different in Grp2 and Grp3 vs Grp1, although DCGF risk was significantly lower in Grp3 vs Grp1 in the DD cohort (HR=0.54; 95% CI=0.31,0.94). HR for GF and 95% CI by UNOS region are presented, Table 2.
Conclusion: BEL was used for a small but increasing number of KTx through Dec 2015. Its use was not limited to, but was concentrated in, a single UNOS region. BEL is most often used 'off-label' with lymphocyte depleting agents and/or CNI. Outcomes with BEL appear comparable to CNI based regimens, with significant regional variability.
CITATION INFORMATION: Sanoff S., Ravindra K., Irish W. Belatacept Maintenance Immunosuppression Use and Outcomes in the Real-World Clinical Setting Using OPTN/UNOS Kidney Transplant Registry Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Sanoff S, Ravindra K, Irish W. Belatacept Maintenance Immunosuppression Use and Outcomes in the Real-World Clinical Setting Using OPTN/UNOS Kidney Transplant Registry [abstract]. https://atcmeetingabstracts.com/abstract/belatacept-maintenance-immunosuppression-use-and-outcomes-in-the-real-world-clinical-setting-using-optn-unos-kidney-transplant-registry/. Accessed March 11, 2026.« Back to 2018 American Transplant Congress