Belatacept Conversion: Are Patients BENEFIT-ing
Columbia University Irving Medical Center, New York, NY
Meeting: 2020 American Transplant Congress
Abstract number: 115
Keywords: Graft failure, Immunosuppression, Nephrotoxicity, Renal function
Session Information
Session Name: Novel Immunosuppression: Belatacept
Session Type: Oral Abstract Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:45pm
 Presentation Time: 4:15pm-4:27pm
Presentation Time: 4:15pm-4:27pm
Location: Virtual
*Purpose: Belatacept is increasingly used in renal transplant recipients experiencing adverse effects from calcineurin inhibitors(CNI) despite limited medium- and long- term data on safety and efficacy. We reviewed our single center data on patients converted to belatacept with a focus on graft outcomes and complication rates.
*Methods: We reviewed our transplant database at CUIMC to identify all patients transitioned from a CNI- to belatacept-based regimen. We collected demographics, donor type, reason for conversion, creatinine values over time, and post-conversion complications.
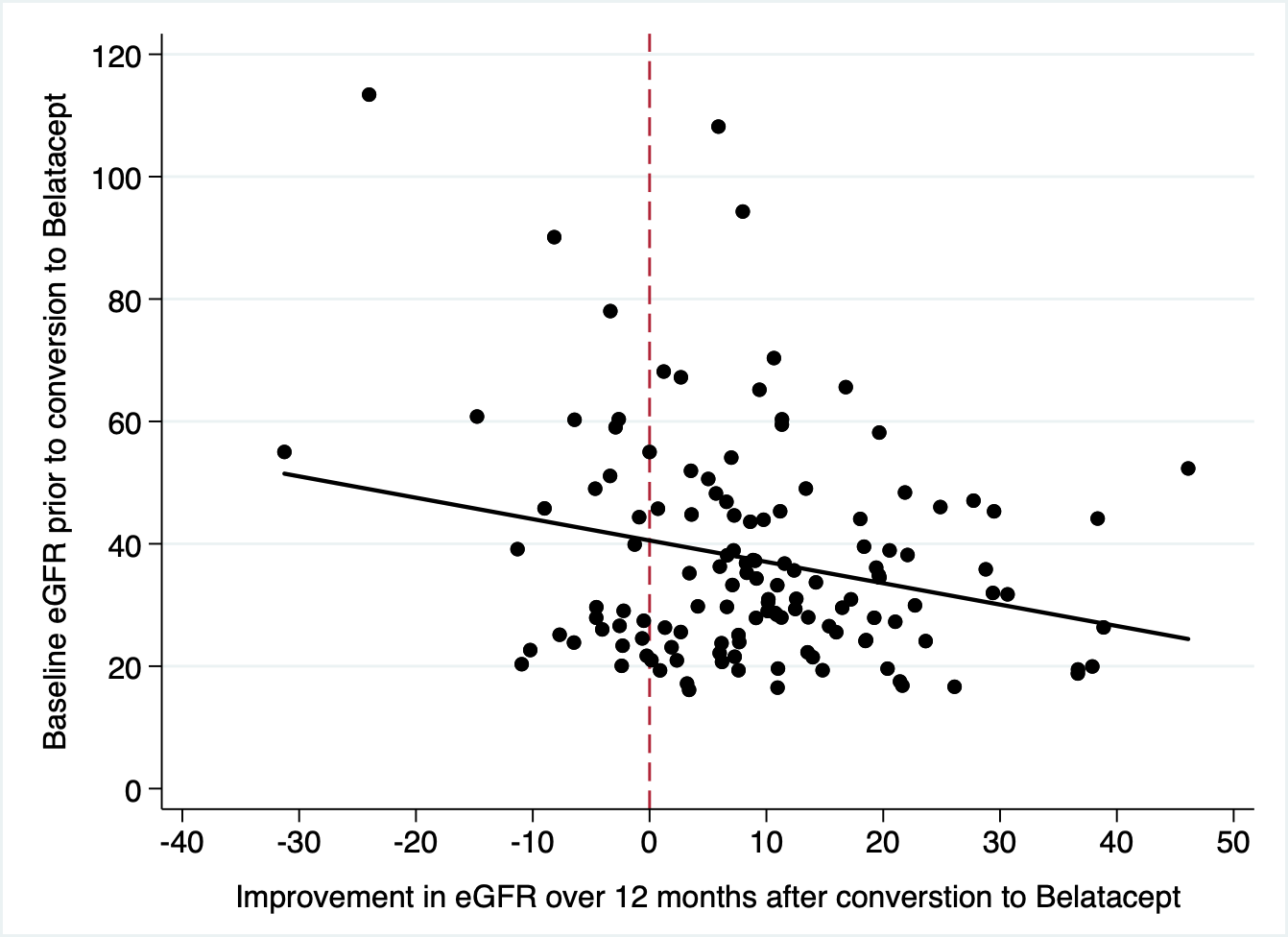
*Results: Since 2013, we have converted 280 patients, 40% of whom were female, at a median age of 52 yrs (IQR- 39-61). 163 had received kidneys from deceased donors. All but 2 patients had received antibody induction therapy (antithymocyte globulin-191, alemtuzumab 75, basiliximab 12). Reasons for conversion were graft dysfunction (212), neurotoxicity (45) thrombotic microangiopathy (20), and other (5). Patients were a median 250 (IQR 85-981) days post-transplant and followed a median 538 (IQR 169-1146) days. Excluding 6 patients with primary nonfunction, the starting creatinine=2.97 (SD=1.9), improving to 1.9(SD=0.9) at 1 yr, and 1.6(SD=0.73) at 3 yrs. Estimated GFR(eGFR) improved in 77% of patients by 3 months. Generally, lower eGFR at the time of conversion predicted greater absolute improvements in renal function.(figure 1) Unfortunately, 47(17%) patients rejected after conversion– 46 cellular rejections (including 7- 2A, 1- 2B, and 1- grade 3), and 1 chronic AMR — at a mean 231 days after conversion. 19(7% overall) had recurrent rejections, and 7 rejecters ultimately lost the allograft from chronic rejection. CMV infection occurred in 23 patients, including 3 cases of CMV retinitis. 28 patients developed BK viremia. 4 patients were diagnosed with lymphoma, and 6 patients developed other malignancies. In addition to 17 deaths and 28 graft failures, 32(11%) discontinued belatacept for lack of efficacy(8), infection(7), lymphoma(3), patient preference (12), or other(2).
*Conclusions: Belatacept has been an important addition to our transplant arsenal, leading to an improvement in graft function in the majority of patients. We should be wary of the high rejection rates, infectious complications, and patient inconvenience when transitioning to this regimen.
To cite this abstract in AMA style:
Crew R, Mohan S, Patel SS. Belatacept Conversion: Are Patients BENEFIT-ing [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/belatacept-conversion-are-patients-benefit-ing/. Accessed March 9, 2026.« Back to 2020 American Transplant Congress