Azole Use and Fungal Pathogens in Cystic Fibrosis versus Non-Cystic Fibrosis Lung Transplant Recipients
1Internal Medicine, Cleveland Clinic Foundation, Cleveland, OH, 2Infectious Disease, Cleveland Clinic Foundation, Cleveland, OH, 3Infectious Disease, Spectrum Health Infectious Disease, Grand Rapids, MI
Meeting: 2019 American Transplant Congress
Abstract number: C336
Keywords: Fungal infection, Lung transplantation, Prophylaxis
Session Information
Session Name: Poster Session C: Lung: All Topics
Session Type: Poster Session
Date: Monday, June 3, 2019
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall C & D
*Purpose: Cystic fibrosis (CF) patients exhibit unique pharmacokinetic principles. Azole antifungals show marked inter- and intra-patient variability. We aimed to improve itraconazole (ITR) use and determine the association with post-transplant fungal pathogen identification in CF lung transplant recipients (LTR) compared to non-CF.
*Methods: We conducted a retrospective cohort study of adult LTR from 2012-2017.
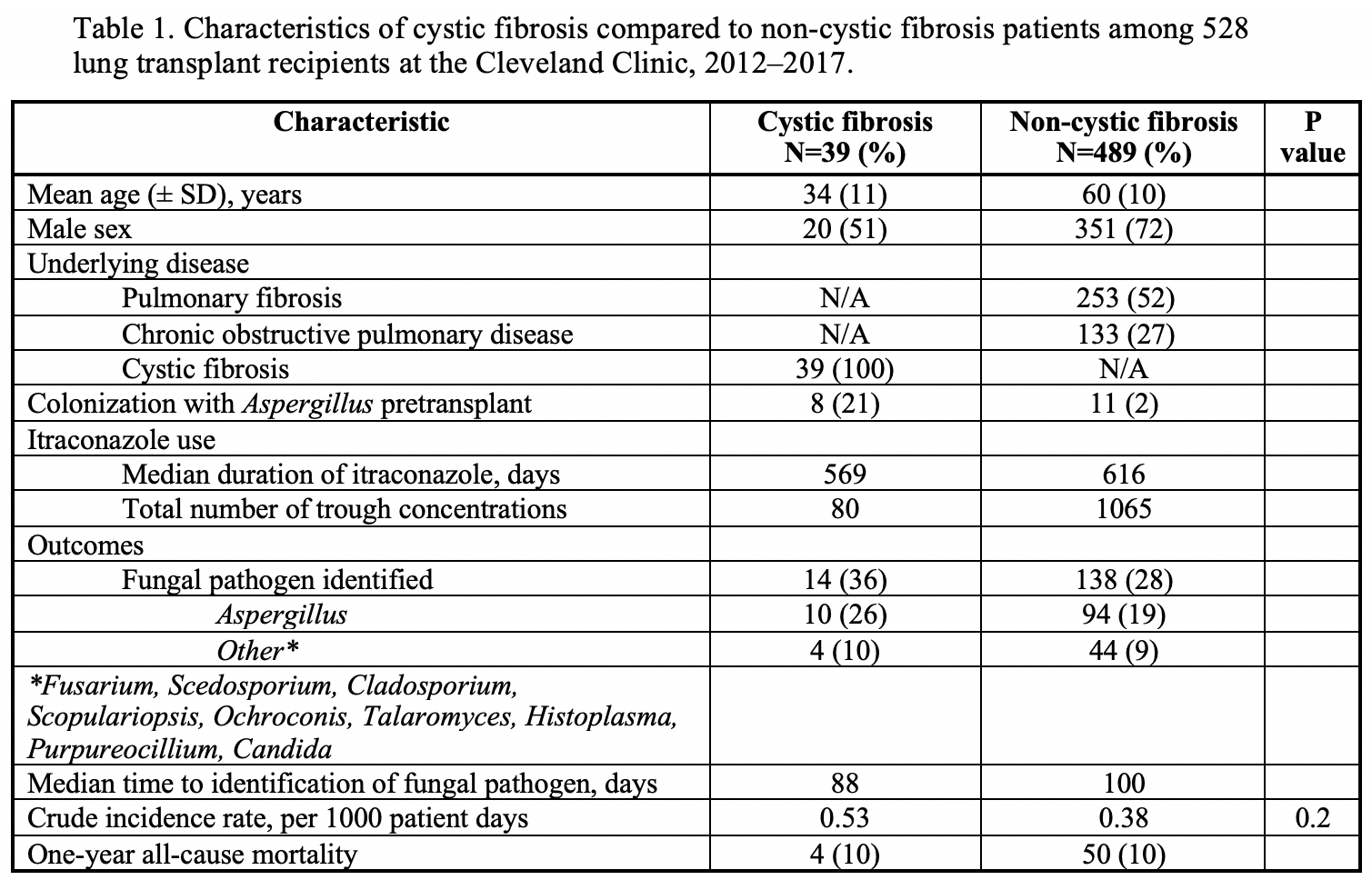
*Results: We identified 528 LTR, 39 CF LTR on 26,341 days of ITR who underwent 80 serum trough concentration measurements and 489 non-CF LTR on 363,226 days of ITR who underwent 1,065 serum trough concentration measurements. All CF patients and 481/489 (98%) non-CF LTR initially received 200 mg per day as suspension. Proton pump inhibitors were used in 34/39 (87%) of the CF patients and 450/489 (92%) of the non-CF patients. We compared ITR levels between the 2 groups. 56% of the CF LTR had initial levels <0.5 (level obtained after a median of 22 days of ITR) compared with 32% for non-CF (p<0.01). Only 8/19 (42%) CF LTR had their dose increased. At later points, significant differences in the ITR levels remained: for CF LTR, 50% had ITR level <0.5 vs. 30% for non-CF at 2ndtime point, and 56 vs. 30% at 3rd. We next evaluated BAL fluid studies to compare fungal pathogens. 14/39 (36%) CF LTR had fungal pathogens identified a median of 88 days after transplant: 10 Aspergillus, 1 Scedosporium, and 3 other. 138/489 (28%) non-CF had fungal pathogens identified a median of 100 days after transplant: 94 Aspergillus, 5 Purpureocillium, 3 Fusarium, 3 Alternaria, 1 Scedosporium, 1 Scopulariopsis, 1Ochroconis gallopavum.
*Conclusions: CF is a significant risk factor for sub-therapeutic ITR trough concentrations post lung transplant. The risks of exposure to such low ITR levels need to be determined. One possible risk involves development of invasive fungal infection. Although in our study there were more fungal pathogens identified in BAL fluid analysis of CF LTR, the difference between CF and non-CF LTR did not reach statistical significance. Dose escalation in CF LTR with close monitoring appears needed to improve prophylaxis in this vulnerable population with unique pharmacokinetic principles at risk of invasive fungal infection.
To cite this abstract in AMA style:
Canosa FJMarco, Brizendine K, Hassouna H. Azole Use and Fungal Pathogens in Cystic Fibrosis versus Non-Cystic Fibrosis Lung Transplant Recipients [abstract]. Am J Transplant. 2019; 19 (suppl 3). https://atcmeetingabstracts.com/abstract/azole-use-and-fungal-pathogens-in-cystic-fibrosis-versus-non-cystic-fibrosis-lung-transplant-recipients/. Accessed February 15, 2026.« Back to 2019 American Transplant Congress