Assessing Proportion of Days Covered (PDC) of Mycophenolate (MCP) Prescriptions in Post Kidney Transplant (KT) Recipients as a Predictor of Clinical Outcomes
K. Sandlin1, T. Sam1, N. Wilson1, A. Yango2, B. Fischbach3, A. Dao1
1Pharmacy, Baylor University Medical Center, Dallas, TX, 2Baylor All Saints Medical Center, Dallas Nephrology Associates, Fort Worth, TX, 3Baylor University Medical Center, Dallas Nephrology Associates, Dallas, TX
Meeting: 2022 American Transplant Congress
Abstract number: 1614
Keywords: Graft failure, Mycophenolate mofetil, Rejection, Risk factors
Topic: Clinical Science » Ethics » 22 - Psychosocial and Treatment Adherence
Session Information
Session Name: Psychosocial and Treatment Adherence
Session Type: Poster Abstract
Date: Tuesday, June 7, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Nonadherence to medications following KT is a risk factor for adverse outcomes. PDC is an objective measure of adherence obtained through retail prescription records and is calculated by the number of days covered by a medication in a specified time period. An 80% cut-off has been reported in the literature as a threshold that stratifies adherent and non-adherent patients. The aim of this study was to assess the impact of adherence as described by PDC of MCP on post-KT outcomes.
*Methods: This was a retrospective chart review conducted via the pharmacy dispensing and health record systems. Recipients who received a KT between 03/2020-06/2021 and persistently filled MCP at a health system’s retail pharmacy between 03/2020-12/2021 were included. Persistent filling was defined as 1 MCP fill within 15 days post-discharge from KT and at least 3 fills within the observation period of 180 days. Recipients who filled MCP at an outside pharmacy or received medications through a patient assistance program were excluded. Donor/recipient characteristics, induction agents, and clinical outcomes were collected. Discharge date, MCP prescription data, and sold dates were used to calculate PDC at 180 days following discharge. A timeline was created for each day in the observation period with adjustments to account for hospital readmissions and dose changes. Recipients were strategized into two cohorts: PDC <80% and PDC >80%.
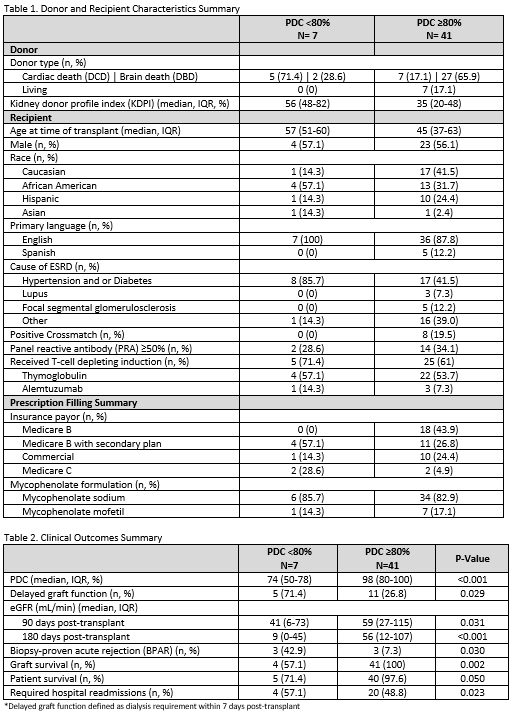
*Results: Forty-eight recipients were included in data analysis (n=7 in PDC < 80%, n=41 in PDC ≥ 80%). Table 1 summarized donor/recipients characteristics, induction therapies, and prescription details. Recipients in PDC <80% cohort received more kidneys from DCD donors and donors with higher KDPI. Those in PDC >80% cohort had a higher proportion with a positive crossmatch and PRA >50%. PDC and post KT outcomes were included in Table 2. Median PDC at 180 days post discharge were 74% and 98% (P <0.001). KT recipients with PDC <80% had significantly lower eGFR at 90- and 180- day post-transplant (P=0.031 & <0.01 respectively), higher rate of BPAR (P=0.03), and lower graft survival (P=0.002).
*Conclusions: In our diverse KT patient sample, nonadherence to medications, defined by a PDC <80% for MCP, is associated with worsened patient and graft outcomes. Utilizing PDC as an indicator of nonadherence may help to identify patients at higher risk of poor outcomes.
To cite this abstract in AMA style:
Sandlin K, Sam T, Wilson N, Yango A, Fischbach B, Dao A. Assessing Proportion of Days Covered (PDC) of Mycophenolate (MCP) Prescriptions in Post Kidney Transplant (KT) Recipients as a Predictor of Clinical Outcomes [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/assessing-proportion-of-days-covered-pdc-of-mycophenolate-mcp-prescriptions-in-post-kidney-transplant-kt-recipients-as-a-predictor-of-clinical-outcomes/. Accessed March 7, 2026.« Back to 2022 American Transplant Congress