An Initial Analysis of Baseline Levels of Dd-cfdna After Pancreas Transplantation: A Prospective Study from High-Volume Centers in the United States
1University of Maryland School of Medicine, Baltimore, MD, 2University of Wisconsin School of Medicine, Madison, WI, 3Georgetown University School of Medicine, Washington, DC
Meeting: 2022 American Transplant Congress
Abstract number: 1146
Keywords: Genomic markers, Infection, Kidney/pancreas transplantation, Rejection
Topic: Clinical Science » Pancreas » 65 - Pancreas and Islet: All Topics
Session Information
Session Name: Pancreas and Islet: All Topics
Session Type: Poster Abstract
Date: Sunday, June 5, 2022
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Donor-derived cell-free DNA (dd-cfDNA) testing, which has been validated for monitoring kidney transplant (KTx) rejection, may provide an alternative to pancreas biopsy that prevents morbidity and graft loss. We determined, for the first time, dd-cfDNA levels in pancreas transplant (PTx) recipients with and without rejection.
*Methods: Patients undergoing PTx were enrolled. Dd-cfDNA levels were collected monthly through month 12 and quarterly thereafter and were assessed in context of patient creatinine, biopsy, and demographics.
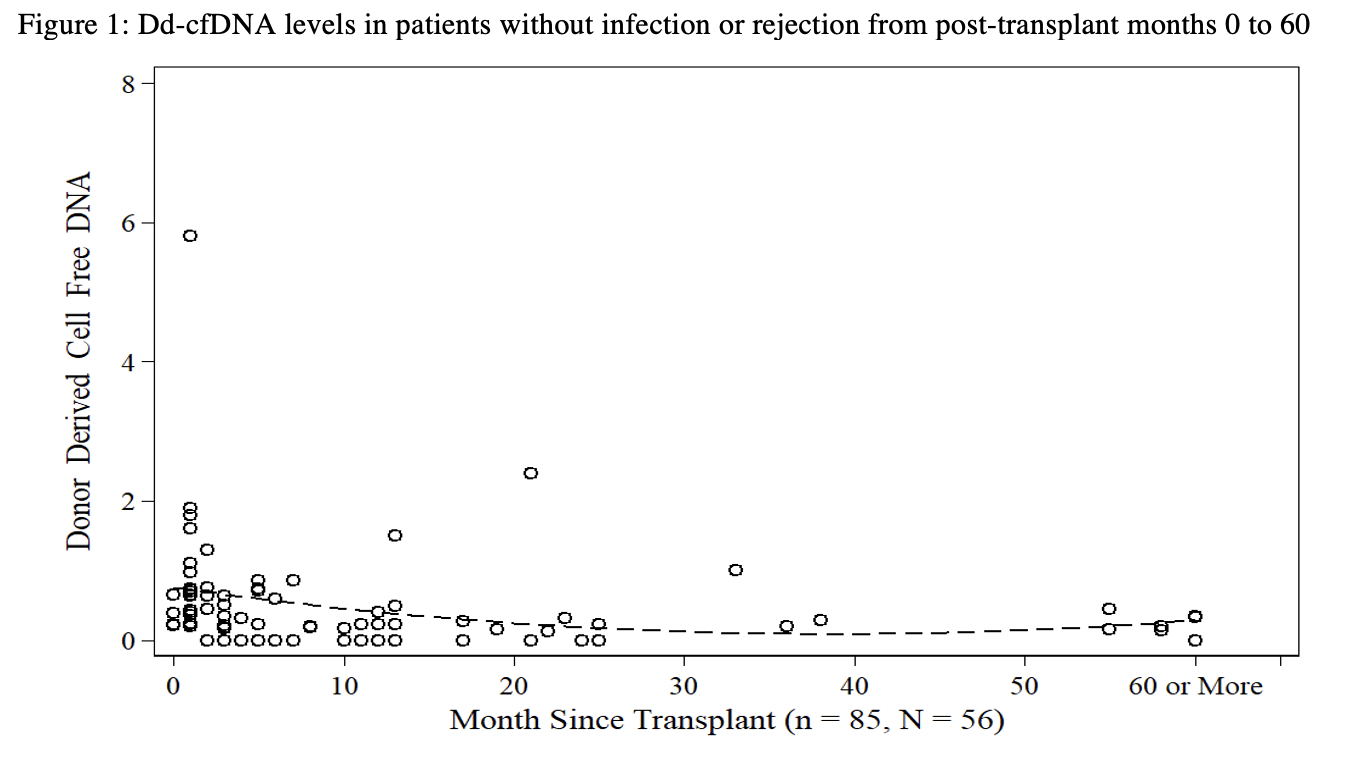
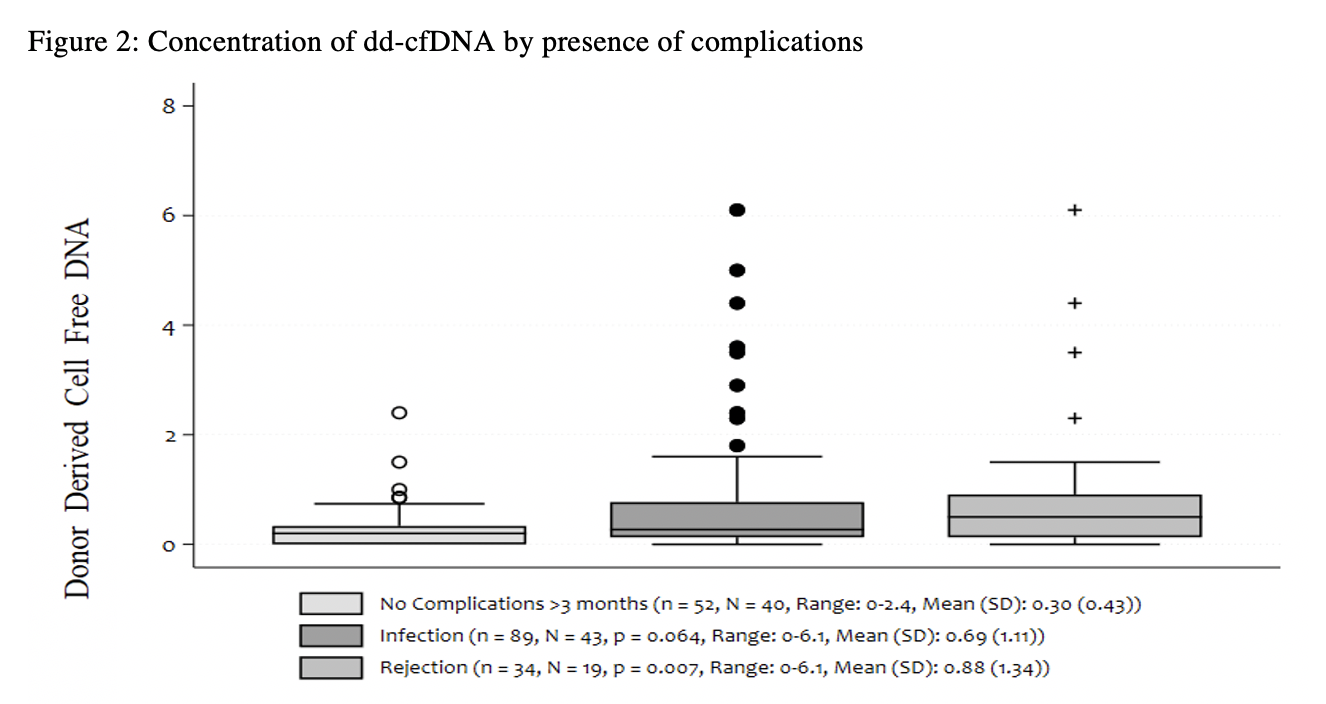
*Results: n=182 dd-cfDNA samples were collected from N=78 patients up to 132 months post-PTx. Mean and median dd-cfDNA levels in all subjects were 0.60±0.9% and 0.28%, respectively. Mean and median dd-cfDNA levels of subjects without rejection or infection were 0.52±0.8% and 0.28% [N=56, n=87], and peaked at 1.03±0.7% and 0.74% within 1-month post-PTx. Mean and median dd-cfDNA levels of subjects with suspicion of rejection were 0.88±1.3% and 0.50% [N=19, n=34]. Mean and median dd-cfDNA levels of subjects at time of infection were 0.69±1.1% and 0.27% [N=43, n=89]. Figure 1 shows dd-cfDNA trends without rejection or infection, revealing an initial elevation in dd-cfDNA <3 months post-PTx (mean 0.85±1.0%, median 0.63%), followed by stabilization >3 months post-PTx (mean 0.3±0.4%, median 0.20). Figure 2 compares dd-cfDNA during rejection, infection and no known complications following stabilization >3 month post-PTx.
*Conclusions: The mean dd-cfDNA level for PTx recipients is <1.0%, consistent with the published KTx rejection threshold of >1.0%. Early levels of dd-cfDNA (>1.0% at 1 month) are transiently higher than later measurements. PTx dd-cfDNA elevation also correlates with rejection and infection. dd-cfDNA is a promising biomarker for PTx immune and infectious complications. Additional data are needed to define a true rejection threshold.
To cite this abstract in AMA style:
Yoo A, Qian I, Riedel A, Kibria G, Bartosic A, Soltani R, Nduanya O, Omidi M, Odorico JS, Abrams PL, Cooper M, Bromberg JS, Scalea JR. An Initial Analysis of Baseline Levels of Dd-cfdna After Pancreas Transplantation: A Prospective Study from High-Volume Centers in the United States [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/an-initial-analysis-of-baseline-levels-of-dd-cfdna-after-pancreas-transplantation-a-prospective-study-from-high-volume-centers-in-the-united-states/. Accessed February 24, 2026.« Back to 2022 American Transplant Congress