Amiodarone Use Increases Morbidity Not Mortality in Pediatric Heart Transplant Recipients
Pediatric Cardiology, St.Louis Children's Hospital, St. Louis, MO.
Meeting: 2018 American Transplant Congress
Abstract number: B33
Keywords: Heart transplant patients, Morbidity, Mortality, Pediatric
Session Information
Session Name: Poster Session B: Heart and VADs: All Topics
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background: Amiodarone is commonly used in pediatric and adult patients with end-stage heart failure and its use is associated with increased 1-year post-transplant mortality in adults. The objectives of our study were to evaluate effects of pre-transplant amiodarone use on post-transplant morbidity and mortality in pediatric recipients.
Methods: The ISHLT registry was used to identify pediatric (≤ 18 year) heart transplant recipients from 1986-2015 treated with amiodarone (group 1) and were compared to recipients treated with other AA (group 2) or no AA (control). Postoperative morbidity and mortality were compared between the groups propensity matched for age, gender, presence of congenital heart disease, and year of transplant.
Results: Pediatric heart recipients on amiodarone and other AA at the time of transplant were 8.6% and 10% respectively. AA use (amiodarone or other AA) was more common in subjects with dilated or hypertrophic cardiomyopathy (p<0.001), history of near sudden death (p<0.001), as well as those with an ICD (p<0.001), VAD (p<0.001), inhaled NO (p=0.02), or ECMO (p=0.04) at time of transplant compared to those on no AA (control). The propensity matched survival analysis revealed no difference in 1-year mortality. 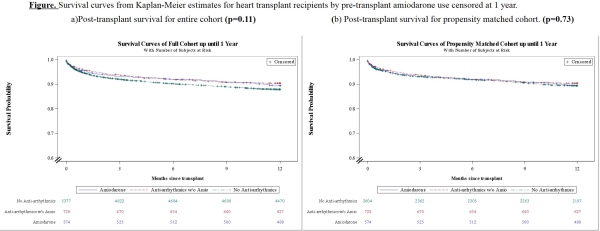 However, amiodarone treated patients were more likely to have graft failure from primary graft dysfunction (11.5 vs. 5 in group 2 vs. 6.5% in controls, p=0.02) and pacemaker placement before (2.5 vs. 1.5 in group 2 vs. 1.2% in controls, p=0.03) and after hospital discharge (2.3 vs. 0 in group 2 vs. 1.2% in controls, p=0.02).
However, amiodarone treated patients were more likely to have graft failure from primary graft dysfunction (11.5 vs. 5 in group 2 vs. 6.5% in controls, p=0.02) and pacemaker placement before (2.5 vs. 1.5 in group 2 vs. 1.2% in controls, p=0.03) and after hospital discharge (2.3 vs. 0 in group 2 vs. 1.2% in controls, p=0.02).
Inferences: Pediatric heart transplant recipients are frequently treated with AA prior to transplant. Though use of AA was not observed to increase 1- year mortality, amiodarone was associated with increased post-transplant morbidity which was not seen with the use of other anti-arrhythmics.
CITATION INFORMATION: Amdani S., Dalal A., Simpson K., Scheel J., Abarbanell A., Eghtesady P., Canter C., Castleberry C. Amiodarone Use Increases Morbidity Not Mortality in Pediatric Heart Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Amdani S, Dalal A, Simpson K, Scheel J, Abarbanell A, Eghtesady P, Canter C, Castleberry C. Amiodarone Use Increases Morbidity Not Mortality in Pediatric Heart Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/amiodarone-use-increases-morbidity-not-mortality-in-pediatric-heart-transplant-recipients/. Accessed July 15, 2025.« Back to 2018 American Transplant Congress
