Allogeneic Mesenchymal Stem Cells as Induction Therapy Are Safe and Feasible in Renal Allografts: Pilot Results of a Multicenter Randomized Controlled Trial
1Third Affiliated Hospital, Sun Yat-sen University, Guangzhou, China
2Zhujiang Hospital, Southern Medical University, Guangzhou, China
3Second Affiliated Hospital, Guangzhou Traditional Chinese Medicine University, Guangzhou, China.
Meeting: 2018 American Transplant Congress
Abstract number: D253
Keywords: Graft function, Kidney transplantation, Rejection, Stem cells
Session Information
Session Name: Poster Session D: Stem Cell, Cellular Therapies and Regenerative Medicine
Session Type: Poster Sessoin
Date: Tuesday, June 5, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background: Delayed graft function (DGF) and acute rejection lead to adverse effects on graft outcomes. Optimal induction intervention should include both renal structure injury repair and immune response suppression. MSCs are considered a candidate to prevent DGF and acute rejection in renal transplantation. This prospective multicenter controlled study aimed to assess clinical value of MSCs as induction therapy to prevent both DGF and acute rejection in renal transplantation.
Methods: 42 recipients were recruited and divided into trial and control groups.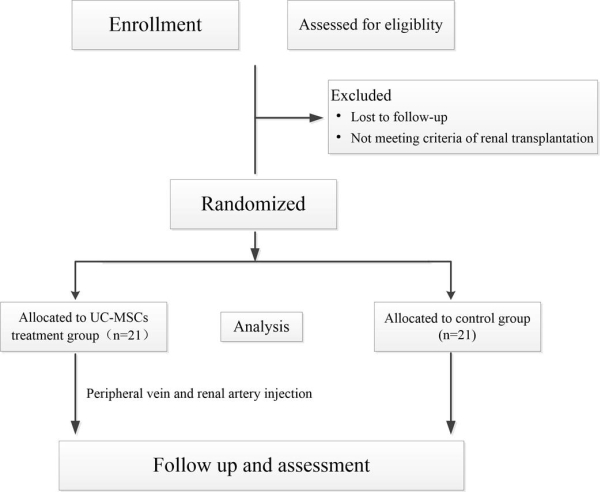 The trial group received 2[times]106/kg umbilical-cord-derived MSCs (UC-MSCs) via peripheral vein before transplantation, and 5[times]106 cells via renal artery during surgery. Incidences of DGF and acute rejection were recorded.Graft and recipient survivals were also evaluated.
The trial group received 2[times]106/kg umbilical-cord-derived MSCs (UC-MSCs) via peripheral vein before transplantation, and 5[times]106 cells via renal artery during surgery. Incidences of DGF and acute rejection were recorded.Graft and recipient survivals were also evaluated.
Results: Treatment with UC-MSC achieved graft and recipient survival rates comparable with non-MSC (p=0.97 and 0.15, respectively). No increase in DGF and acute rejection, were observed (DGF: 9.5% in the MSC group versus 33.3% in non-MSC,p=0.13; acute rejection:14.3% versus 4.8%, p=0.61). Equal eGFRs were found between the two groups (p=0.88). All patients tolerated MSC without adverse clinical effects. Additionally, a multiprobe FISH assay revealed that UC-MSCs administered via renal artery were absent from the recipient's biopsy sample.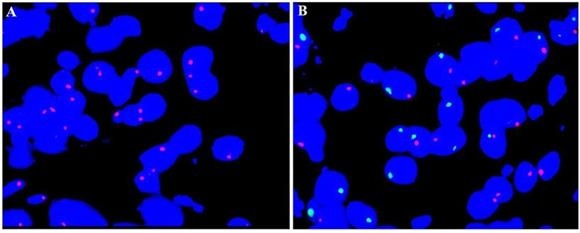
Conclusions: UC-MSCs can be used as clinically feasible and safe induction therapy. Adequate timing and frequency of UC-MSC administration may have a significant effect on graft and recipient outcomes.
CITATION INFORMATION: Sun Q., Han F., Zhao M., Cao R., Zhao D., Huang Z., Hong L., Peng Y., Sun Q. Allogeneic Mesenchymal Stem Cells as Induction Therapy Are Safe and Feasible in Renal Allografts: Pilot Results of a Multicenter Randomized Controlled Trial Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Sun Q, Han F, Zhao M, Cao R, Zhao D, Huang Z, Hong L, Peng Y, Sun Q. Allogeneic Mesenchymal Stem Cells as Induction Therapy Are Safe and Feasible in Renal Allografts: Pilot Results of a Multicenter Randomized Controlled Trial [abstract]. https://atcmeetingabstracts.com/abstract/allogeneic-mesenchymal-stem-cells-as-induction-therapy-are-safe-and-feasible-in-renal-allografts-pilot-results-of-a-multicenter-randomized-controlled-trial/. Accessed February 25, 2026.« Back to 2018 American Transplant Congress
