Alemtuzumab Use in a Single Center Pediatric Heart Transplant Cohort.
1University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO
2Pharmacy, Children's Hospital Colorado, Aurora, CO
3Pediatrics, Division of Cardiology, University of Colorado School of Medicine, Anschutz Medical Campus, Aurora, CO
Meeting: 2017 American Transplant Congress
Abstract number: C102
Keywords: Heart transplant patients, Pediatric
Session Information
Session Name: Poster Session C: Hearts and VADS: All Topics
Session Type: Poster Session
Date: Monday, May 1, 2017
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall D1
Purpose: To describe the use and complications of alemtuzumab (AMB), a CD52 monoclonal antibody, in pediatric heart transplant (HT) patients (pt).
Methods: Retrospective chart review of HT pt at our center who received AMB in a protocolized fashion (1993-2014). Indications for AMB were induction for repeat-HT (RHT) or treatment of severe or refractory acute rejection rejection (AR). Infection and AR events were included if they met Pediatric Heart Transplant Study definitions. Incidence rates (IR) of AR, malignancy, and infection were calculated as the number of events per patient year (pt-yr). Freedom from AR, infection, graft loss and death were analyzed using Kaplan Meier analysis and the log rank test with stratification based on indication for AMB use (AR vs RHT).
Results: There were 27 patient that received 28 treatments of AMB. Indication for AMB was induction for RHT (n=13) and 15 AR events. The median age at first HT and AMB administration were 1.3 [0.2-8.4] and 10.6 [5.4-15.2], respectively. There was a total of 325.4 pt-yr of post-HT follow-up; 191.8 pt-yr pre-AMB and 133.6 pt-yr post-AMB. There were a total of 96 AR events (IR=0.30/pt-yr); 63 pre-AMB (IR=0.33/pt-yr) and 33 post-AMB (IR=0.25/pt-yr). There were a 45 infections post-AMB (IR=0.33/pt-yr) and 2 malignancies (0.015/pt-yr). Table1 shows the results of survival analysis. 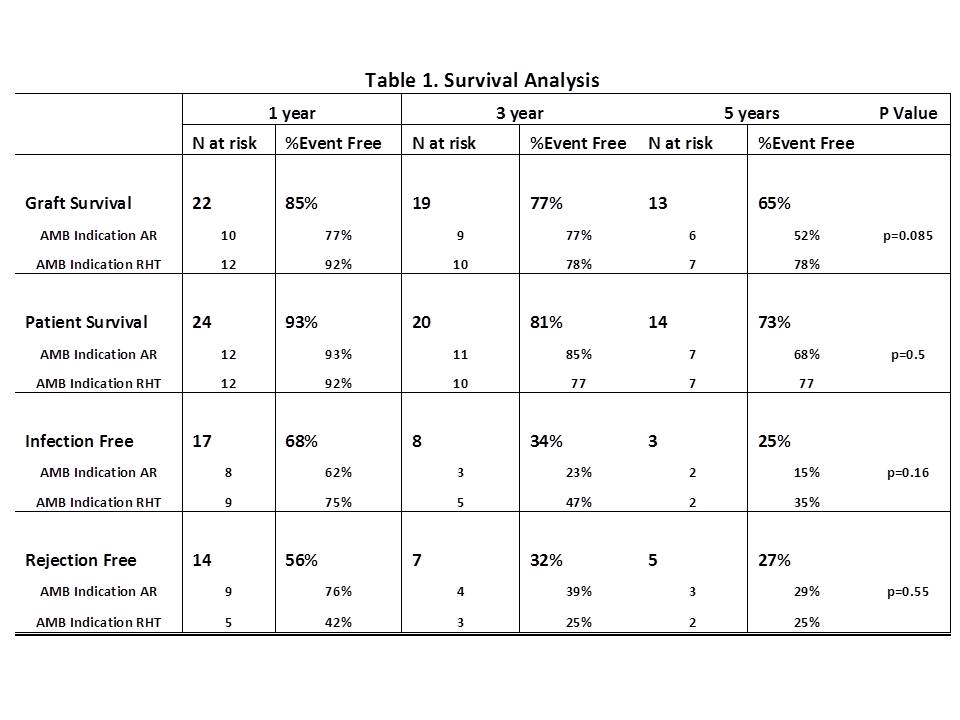 Conclusion: Use of AMB for induction for RHT results is survival that is in line with published outcomes. While there may be slight reduction in the incidence rate of AR after AMB, there is still a relatively low freedom from infection and AR and need for RHT still occurs. Consideration should be given to less noxious treatments such as photopheresis prior to using AMB.
Conclusion: Use of AMB for induction for RHT results is survival that is in line with published outcomes. While there may be slight reduction in the incidence rate of AR after AMB, there is still a relatively low freedom from infection and AR and need for RHT still occurs. Consideration should be given to less noxious treatments such as photopheresis prior to using AMB.
CITATION INFORMATION: Case J, Eshelman J, Booker P, Miyamoto S, Everitt M, Auerbach S. Alemtuzumab Use in a Single Center Pediatric Heart Transplant Cohort. Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Case J, Eshelman J, Booker P, Miyamoto S, Everitt M, Auerbach S. Alemtuzumab Use in a Single Center Pediatric Heart Transplant Cohort. [abstract]. Am J Transplant. 2017; 17 (suppl 3). https://atcmeetingabstracts.com/abstract/alemtuzumab-use-in-a-single-center-pediatric-heart-transplant-cohort/. Accessed July 15, 2025.« Back to 2017 American Transplant Congress
