A Randomized-Controlled Trial Comparing the Efficacy of CYP3A5 Genotype-Based with Bodyweight-Based Tacrolimus Dosing After Living Donor Kidney Transplantation.
1Internal Medicine, Erasmus Medical Centre, Rotterdam, Netherlands
2Hospital Pharmacy, Erasmus Medical Centre, Rotterdam, Netherlands
3Clinical Chemistry, Erasmus Medical Centre, Rotterdam, Netherlands
4Pathology, Erasmus Medical Centre, Rotterdam, Netherlands
5Pathology, Academic Medical Centre, Amsterdam, Amsterdam, Netherlands.
Meeting: 2016 American Transplant Congress
Abstract number: B114
Keywords: Gene polymorphism, Immunosuppression, Kidney
Session Information
Session Name: Poster Session B: Drug Minimization
Session Type: Poster Session
Date: Sunday, June 12, 2016
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Halls C&D
Patients expressing the cytochrome P450 (CYP) 3A5 gene require a higher tacrolimus dose to achieve therapeutic exposure compared with non-expressers. This randomized-controlled study investigated if adaptation of the tacrolimus starting dose according to CYP3A5 genotype increases the proportion of kidney transplant recipients being within the target tacrolimus predose concentration range (10-15 ng/mL) at first steady-state. Two hundred forty living-donor, renal transplant recipients were assigned to either receive a standard, bodyweight-based or a CYP3A5 genotype-based tacrolimus starting dose. At day 3, no difference in the proportion of patients having a tacrolimus exposure within the target range was observed between the standard-dose and genotype-based groups: 37.4% vs. 35.6%, respectively; p = 0.79. The proportion of patients with a sub-therapeutic (i.e. <10 ng/mL) or a supra-therapeutic (i.e. >15 ng/mL) Tac C0 in the two groups was also not significantly different. 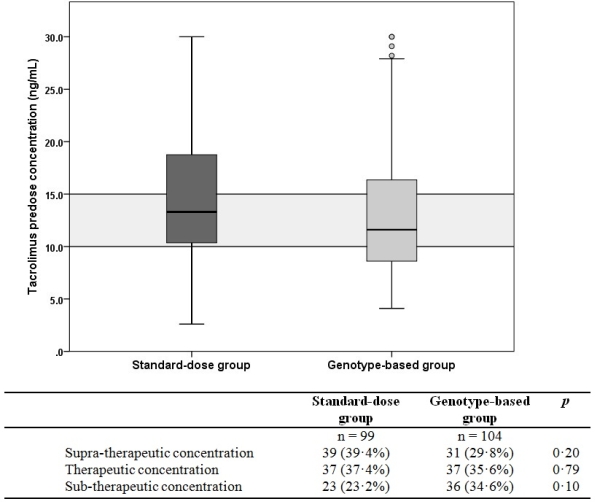 The incidence of acute rejection was comparable between both groups: 10.1% and 11.0% for the standard bodyweight-based and genotype-based groups, respectively; p = 0.82. Pharmacogenetic adaptation of the tacrolimus starting dose does not increase the number of patients having therapeutic tacrolimus exposure early after transplantation and does not lead to improved clinical outcome in a low immunological risk population.
The incidence of acute rejection was comparable between both groups: 10.1% and 11.0% for the standard bodyweight-based and genotype-based groups, respectively; p = 0.82. Pharmacogenetic adaptation of the tacrolimus starting dose does not increase the number of patients having therapeutic tacrolimus exposure early after transplantation and does not lead to improved clinical outcome in a low immunological risk population.
CITATION INFORMATION: Shuker N, Bouamar R, van Schaik R, Clahsen -van Groningen M, Damman J, Baan C, van de Wetering J, Rowshani A, Weimar W, van Gelder T, Hesselink D. A Randomized-Controlled Trial Comparing the Efficacy of CYP3A5 Genotype-Based with Bodyweight-Based Tacrolimus Dosing After Living Donor Kidney Transplantation. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Shuker N, Bouamar R, Schaik Rvan, Groningen MClahsen-van, Damman J, Baan C, Wetering Jvande, Rowshani A, Weimar W, Gelder Tvan, Hesselink D. A Randomized-Controlled Trial Comparing the Efficacy of CYP3A5 Genotype-Based with Bodyweight-Based Tacrolimus Dosing After Living Donor Kidney Transplantation. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/a-randomized-controlled-trial-comparing-the-efficacy-of-cyp3a5-genotype-based-with-bodyweight-based-tacrolimus-dosing-after-living-donor-kidney-transplantation/. Accessed February 23, 2026.« Back to 2016 American Transplant Congress
