A Model for Medical Device Innovation in Transplantation: Using the Biodesign Process to Address the Impact of Warm Ischemic Injury
1University of California San Francisco, San Francisco, CA, 2Stanford University, Palo Alto, CA
Meeting: 2022 American Transplant Congress
Abstract number: 907
Keywords: Bioengineering, Kidney transplantation, Surgical complications, Warm ischemia
Topic: Clinical Science » Organ Inclusive » 69 - Non-Organ Specific:Organ Preservation/Ischemia Reperfusion Injury
Session Information
Session Name: Non-Organ Specific: Organ Preservation/Ischemia Reperfusion Injury
Session Type: Poster Abstract
Date: Saturday, June 4, 2022
Session Time: 5:30pm-7:00pm
 Presentation Time: 5:30pm-7:00pm
Presentation Time: 5:30pm-7:00pm
Location: Hynes Halls C & D
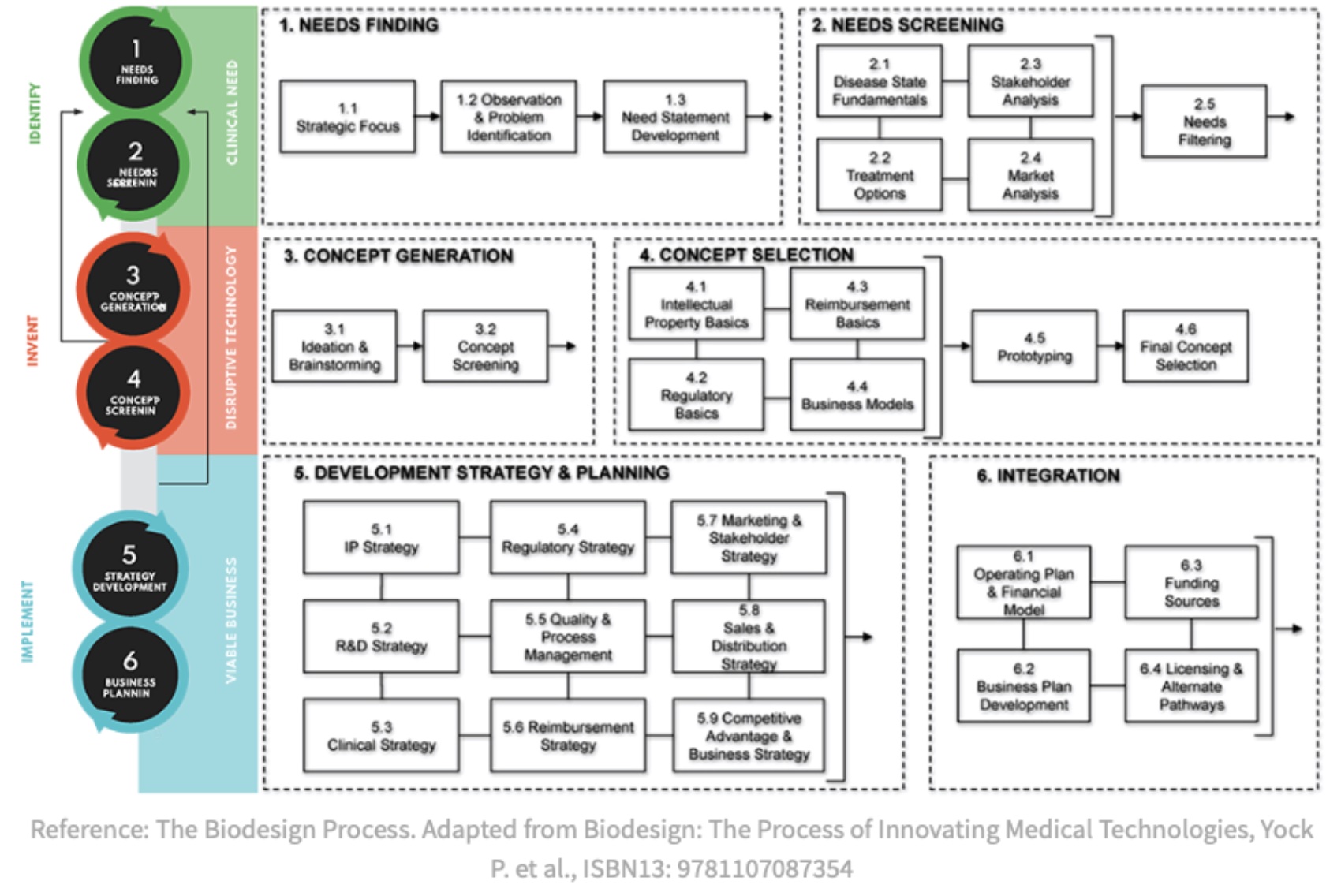
*Purpose: The impact of warming during second warm-ischemia (anastomosis) of kidney transplantation has been increasingly recognized in the literature. The outsized effect on delayed graft function and premature graft failure has led to calls from surgeons and the FDA for a method to prevent warm ischemic injury. The Biodesign process is a formal needs-based process of identification, invention, and implementation for the development of medical-devices. We outline our application of the biodesign-process to transplantation for the development of an intra-operative facilitation device with an incorporated method of temperature regulation to address this clinical need.
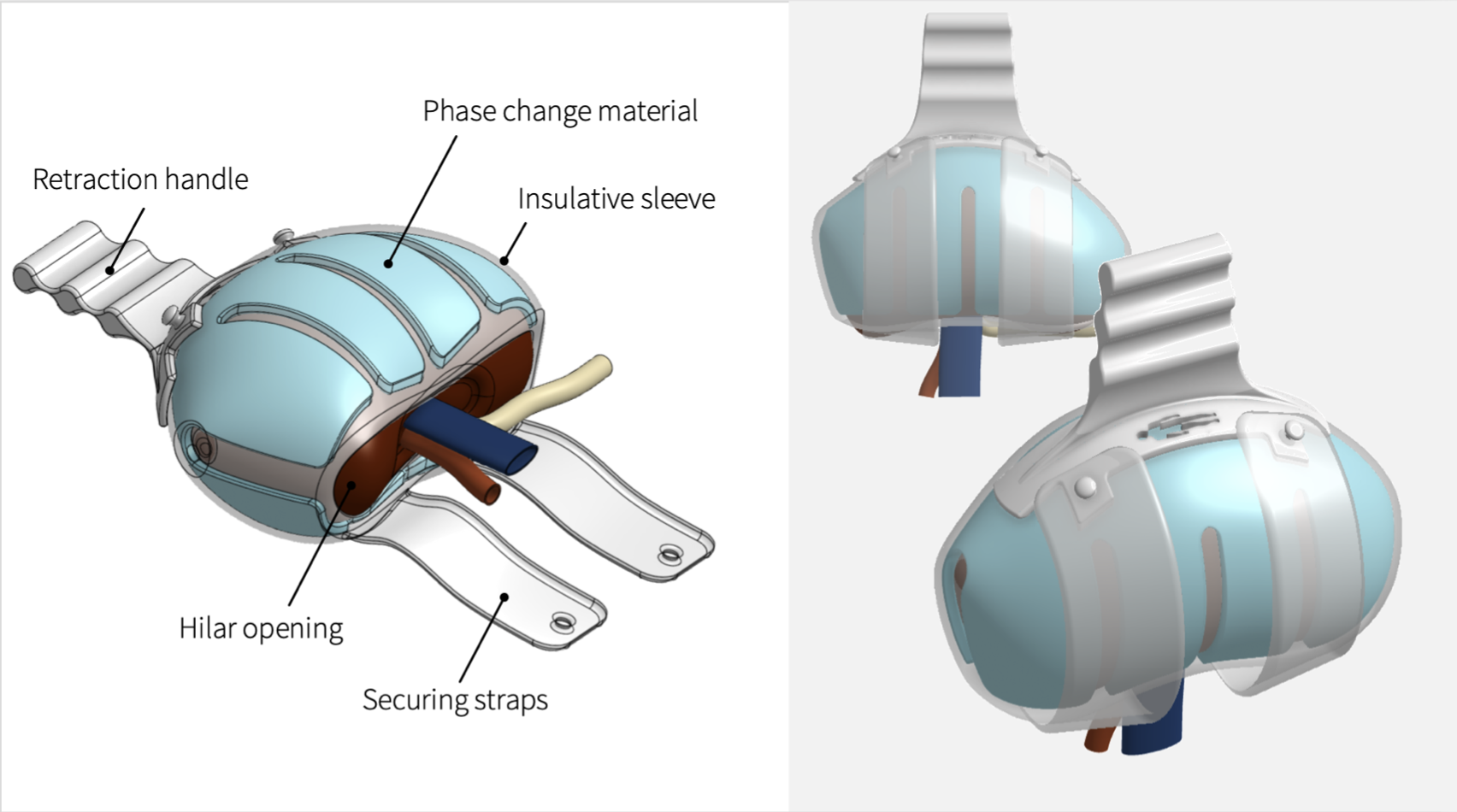
*Methods: We followed the Biodesign process to identify this need through observation, developed a needs statement, then filtered the need against others. A needs-specification document was developed outlining stakeholder value propositions, health-economic value, competition, and existing intellectual property. The next step, invention, resulted in ideation to develop a concept that addressed the need, population, and outcome outlined in our needs statement. Following objective concept selection, a prototype was developed for a device that simplifies anastomosis while preventing kidney warming. A development strategy and business plan were produced as part of the implementation process to commercialize the device.
*Results: A kidney transplantation facilitation device with an incorporated method of temperature regulation was created with a viable commercial model for development. Health-economic and commercial value for this transplant focused device was outlined.
*Conclusions: While the development of transplant-focused medical devices are especially challenging and limited by the relatively small market size, the biodesign process can be replicated to ensure successful development of devices that address clinical needs in the field of transplantation.
To cite this abstract in AMA style:
Hansen K, Wall J. A Model for Medical Device Innovation in Transplantation: Using the Biodesign Process to Address the Impact of Warm Ischemic Injury [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/a-model-for-medical-device-innovation-in-transplantation-using-the-biodesign-process-to-address-the-impact-of-warm-ischemic-injury/. Accessed February 26, 2026.« Back to 2022 American Transplant Congress