A Comprehensive Evaluation of Risk Factors for Pneumocystis Jiroveci Pneumonia in Solid Organ Transplant Recipients: A Systematic Review and Meta-Analysis
N. Permpalung1, V. Kittipibul2, P. Mekraksakit3, P. Rattanawong4, S. Nematollahi1, S. Mehta-Steinke1
1Johns Hopkins University School of Medicine, Baltimore, MD, 2University of Miami/Jackson Memorial Hospital, Miami, FL, 3Texas Tech University Health Sciences, Lubbock, TX, 4Mayo Clinic, Phoenix, AZ
Meeting: 2020 American Transplant Congress
Abstract number: B-177
Keywords: Cytomeglovirus, Pneumonia, Rejection
Session Information
Session Name: Poster Session B: All Infections (Excluding Kidney & Viral Hepatitis)
Session Type: Poster Session
Date: Saturday, May 30, 2020
Session Time: 3:15pm-4:00pm
 Presentation Time: 3:30pm-4:00pm
Presentation Time: 3:30pm-4:00pm
Location: Virtual
*Purpose: Pneumocytis jiroveci pneumonia (PJP) is an important opportunistic fungal infection in solid organ transplant (SOT) recipients that carries a significant morbidity and mortality. Several studies have been conducted to evaluate post-transplant PJP risk factors; however, the results were conflicted and the effect of potential risk factors might be limited due to small sample size.
*Methods: A comprehensive literature search was conducted from inception through August 2019 using MEDLINE, EMBASE and Scopus. All studies evaluating PJP risk factors, including interventional, cohort, case-control and observational studies were included. Pooled odds ratios (OR) with 95% confidence intervals (CI) and the I2 statistic using the random-effects model were calculated.
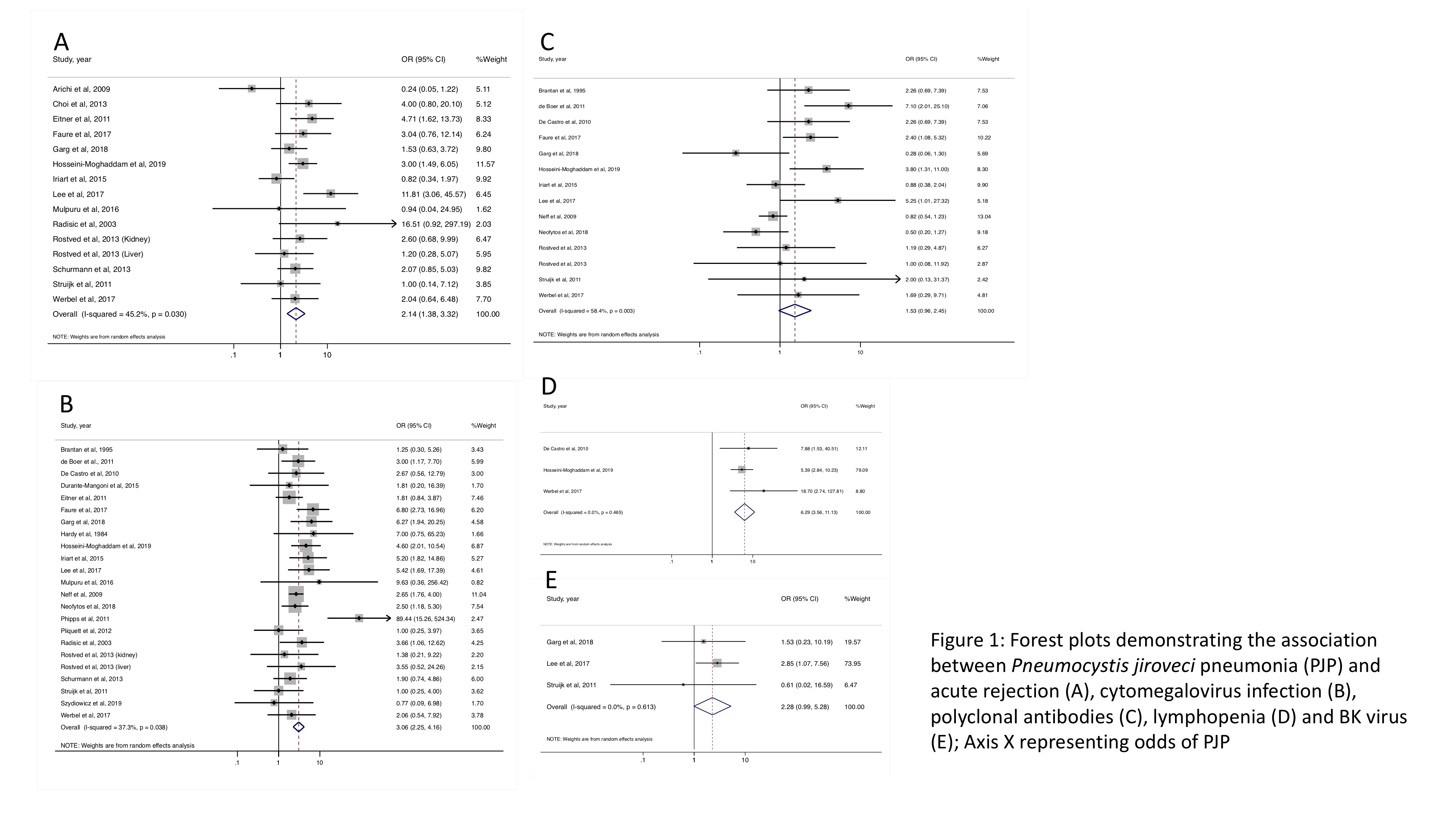
*Results: A total of 27 studies including 39,883 SOT recipients were included. The following factors were associated with PJP development: acute rejection (pooled OR = 2.14, 95% CI [1.38, 3.32], I2 45.2%), cytomegalovirus (CMV) including CMV viremia and CMV disease (pooled OR = 3.06, 95% CI [2.25, 4.16], I2 37.3%) and absolute lymphocyte count less than 500 cells/microliter (pooled OR = 6.29, 95% CI [3.56, 11.13], I2 0%). Polyclonal antibodies (including rabbit anti-thymocyte globulin, thymoglobulin and anti-thymocyte globulin) and BK infection did not significantly increase risk of PJP; however, a trend toward increased PJP risk was observed (pooled OR = 1.53, 95% CI [0.96, 2.45], I2 43.9% and pooled OR = 2.28, 95% CI [0.99, 5.28], I2 0%). Sex, CMV serostatus D+/R-, monoclonal antibody (including alemtuzumab and muromonab-CD3), interleukin-2 inhibitor (including basiliximab and daclizumab), rituximab and methylprednisolone were not associated with developing PJP.
*Conclusions: Acute rejection, CMV and lymphopenia are associated with PJP development, and PJP prophylaxis should be considered in SOT patients with these conditions. Polyclonal antibodies and BK infection may increase the risk of PJP. The absence of significant association between BK and PJP could be due to the small pooled population. We hypothesize that BK infection could indirectly be indicative of a patient’s net state of immunosuppression. Therefore, it may be reasonable to consider PJP prophylaxis in SOT recipients with BK infection or recent polyclonal antibody exposure.
To cite this abstract in AMA style:
Permpalung N, Kittipibul V, Mekraksakit P, Rattanawong P, Nematollahi S, Mehta-Steinke S. A Comprehensive Evaluation of Risk Factors for Pneumocystis Jiroveci Pneumonia in Solid Organ Transplant Recipients: A Systematic Review and Meta-Analysis [abstract]. Am J Transplant. 2020; 20 (suppl 3). https://atcmeetingabstracts.com/abstract/a-comprehensive-evaluation-of-risk-factors-for-pneumocystis-jiroveci-pneumonia-in-solid-organ-transplant-recipients-a-systematic-review-and-meta-analysis/. Accessed February 27, 2026.« Back to 2020 American Transplant Congress