A Comparison of Belatacept Conversion Regimens from Calcineurin Inhibitor-Based Immunosuppression
Cedars-Sinai Medical Center, Los Angeles, CA
Meeting: 2021 American Transplant Congress
Abstract number: 934
Keywords: Calcineurin, Co-stimulation, Immunosuppression, Kidney transplantation
Topic: Clinical Science » Kidney » Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Information
Session Name: Kidney Immunosuppression: Novel Regimens and Drug Minimization
Session Type: Poster Abstract
Session Date & Time: None. Available on demand.
Location: Virtual
*Purpose: Outcomes with varying belatacept conversion protocols from calcineurin inhibitor (CNI)-based immunosuppression have been published but no standard protocol has been established. Beginning in 2012, our conversion protocol consisted of belatacept 5 mg/kg monthly with CNI discontinuation within one month. Given an excess of rejections observed among high-immunologic risk patients converted early after transplant, we later changed our conversion protocol to belatacept 5 mg/kg monthly with spaced weaning of CNI over 3-6 months. Here, we examined outcomes with both regimens.
*Methods: From 2012-2019, 206 kidney transplant patients maintained on CNI were converted to belatacept. Of these, 56 discontinued CNI within 30 days of belatacept initiation and 150 maintained CNI for ≥30 days. Rejection-free and graft survival were compared.
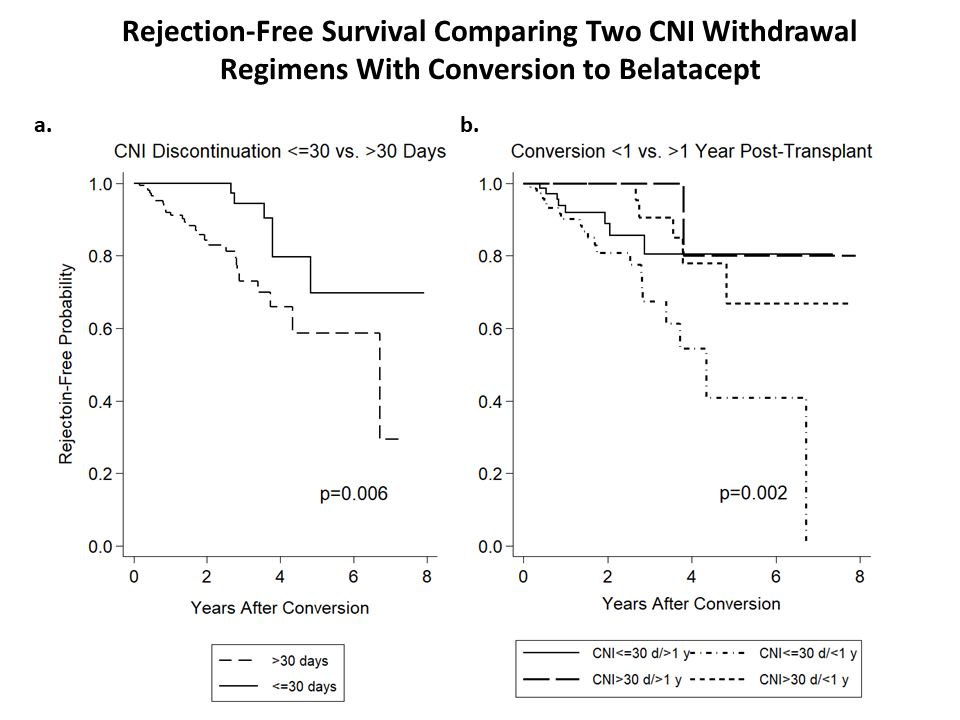
*Results: Baseline characteristics are shown in Table 1. One hundred patients (49%) were converted within 1 year of transplant. Forty-three patients (21%) had historical DSA or DSA at conversion. Among 34 (17%) patients with rejection after conversion over 8 years, there were 30 cases of CMR and 9 cases of ABMR; 23/30 CMR and 6/9 ABMR cases developed among patients converted within 1 year of transplant. Rejections were more frequent in patients who discontinued CNI ≥30 days vs. <30 days (Fig. 1a; log-rank p=0.006) and among patients converted within the first transplant year in both CNI withdrawal regimens (Fig. 1b; log-rank p=0.002). In a multivariable model, CNI discontinuation ≥30 days and conversion within the first transplant year were associated with rejection, whereas sensitization (cPRA≥30%, historical/existing DSA) was not. Graft survival was similar among patients who discontinued CNI ≥30 days vs. <30 days but was lower among patients who underwent conversion within the first transplant year (41% vs. 79%; log-rank p=0.03).
*Conclusions: There were more rejections among patients who discontinued CNI ≥30 days vs. <30 days, although this may be due to selection bias; at a minimum, our data does not indicate an advantage of spaced weaning of CNI more than 30 days after conversion compared to more rapid CNI withdrawal. Belatacept conversion within the first transplant year should be done cautiously.
To cite this abstract in AMA style:
Huang E, Vo A, Peng A, Najjar R, Sethi S, Jordan S. A Comparison of Belatacept Conversion Regimens from Calcineurin Inhibitor-Based Immunosuppression [abstract]. Am J Transplant. 2021; 21 (suppl 3). https://atcmeetingabstracts.com/abstract/a-comparison-of-belatacept-conversion-regimens-from-calcineurin-inhibitor-based-immunosuppression/. Accessed March 3, 2026.« Back to 2021 American Transplant Congress