A Comparison of Alemtuzumab versus Antithymocyte Globulin Induction in High-Risk, Non-Sensitized Renal Transplant Recipients
University of Illinois Hospital and Health Sciences System, Chicago.
Meeting: 2018 American Transplant Congress
Abstract number: B139
Keywords: African-American, Induction therapy, Kidney transplantation
Session Information
Session Name: Poster Session B: Kidney Immunosuppression: Induction Therapy
Session Type: Poster Session
Date: Sunday, June 3, 2018
Session Time: 6:00pm-7:00pm
 Presentation Time: 6:00pm-7:00pm
Presentation Time: 6:00pm-7:00pm
Location: Hall 4EF
Background: Lymphocyte depleting induction is routinely utilized in high immunologic risk renal transplant (RTx). African Americans (AA) have a relatively higher risk of rejection regardless of sensitization. Little data compares induction strategies in this population. The purpose of this study is to compare outcomes of alemtuzumab vs. rabbit anti-thymocyte globulin (rATG) induction in high-risk, non-sensitized AA RTx recipients.
Methods: This was a prospective, randomized study with enrollment from 10/7/13 to 3/2/17. Patients were randomized to induction with rATG 1.5 mg/kg (ideal weight) for 5 doses or alemtuzumab 30 mg. Adult AA patients were included. Highly sensitized patients (i.e. PRA > 10%, ABO incompatible, positive crossmatch) were excluded. All patients received methylprednisolone 500mg and a 5-day steroid withdrawal taper plus tacrolimus and mycophenolic acid maintenance. Primary outcome was biopsy proven acute rejection (BPAR) at 1 year post-RTx. Secondary outcomes included eGFR, leucopenia, and infections.
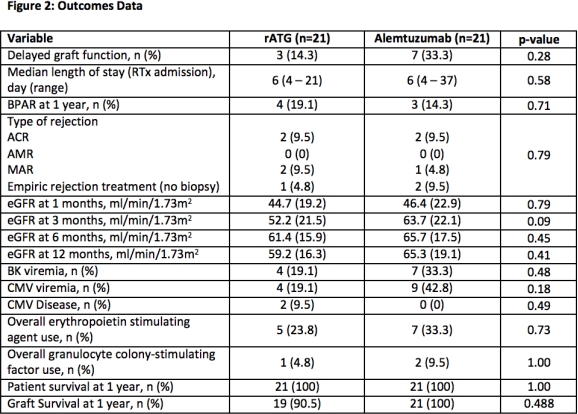
Results: 42 patients were enrolled and completed the study. Overall, patients were male (69%) and received a deceased donor RTx (66.7%) (Figure 1). There was no difference in BPAR (19.1% vs. 14.3%, p=0.71) at 1 year (Figure 2). Patients had similar eGFR at all time points. There were no differences in CMV or BKV. Use of erythropoietin stimulating agents and granulocyte colony-stimulating factors were similar.
Conclusions: These results provide neccessary safety and efficacy data comparing the use of lymphocyte depleting agents in AA RTx recipients and demonstrate that either agent can be effectively utilized. Larger population studies with longer follow-up are needed to determine nuanced differences in lymphocyte depleting induction strategies. 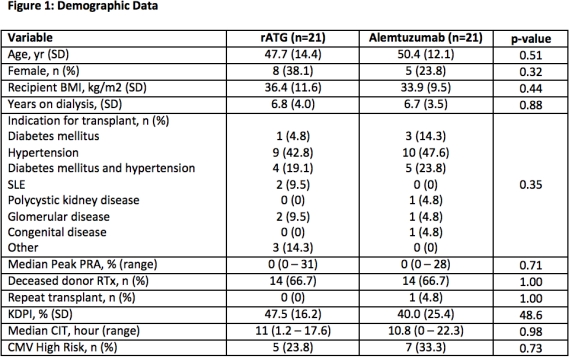
CITATION INFORMATION: Patel S., Lichvar A., Benedetti E., West-Thielke P. A Comparison of Alemtuzumab versus Antithymocyte Globulin Induction in High-Risk, Non-Sensitized Renal Transplant Recipients Am J Transplant. 2017;17 (suppl 3).
To cite this abstract in AMA style:
Patel S, Lichvar A, Benedetti E, West-Thielke P. A Comparison of Alemtuzumab versus Antithymocyte Globulin Induction in High-Risk, Non-Sensitized Renal Transplant Recipients [abstract]. https://atcmeetingabstracts.com/abstract/a-comparison-of-alemtuzumab-versus-antithymocyte-globulin-induction-in-high-risk-non-sensitized-renal-transplant-recipients/. Accessed February 25, 2026.« Back to 2018 American Transplant Congress
