6-Month Universal CMV Prophylaxis and Neutropenia in Kidney Transplant Patients Induced with Alemtuzumab: A Single Center Retrospective Study
Northwestern Univ, Chicago
NU Transplant Outcomes Research Collaboration, Chicago
Anolinx, LLC, Salt Lake City
Genentech, Inc, South San Francisco
Meeting: 2013 American Transplant Congress
Abstract number: A583
Background: Cytomegalovirus remains the leading cause of infectious morbidity in transplant recipients. Universal prophylaxis of kidney transplant patients with oral valganciclovir (valGCV) has reduced the incidence of CMV but is associated with neutropenia as the most common adverse effect. The impact of 6-month universal prophylaxis with valGCV on neutropenia has not been studied in the setting of alemtuzumab induction.
Methods: Electronic medical records of 1,718 recipients of kidney transplants performed at Northwestern Memorial Hospital 1/1/2006 – 10/31/2011 who received both alemtuzumab induction and valGCV prophylaxis were retrospectively reviewed, after IRB approval. Data were collected on patients’ demographics and clinical characteristics as well as data on absolute neutrophil count and G-CSF utilization. Analyses were conducted using descriptive statistics and Kaplan-Meier curves.
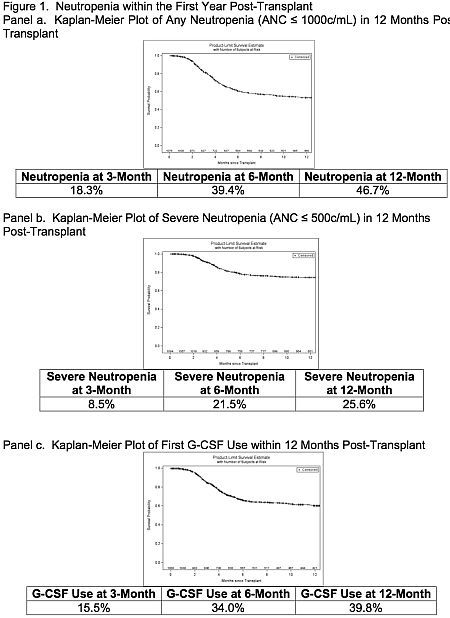
Results: Key demographic and clinical data are in table 1; average duration of valGCV was 177d. At 1 year, 46.7% of patients developed neutropenia (ANC ≤1000 c/mL), 25.6% developed severe neutropenia (ANC ≤ 500 c/mL) and 39.8% required at least a single dose of G-CSF (see figure 1).
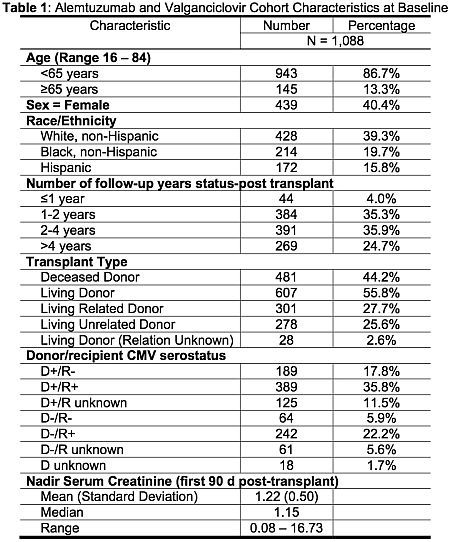
Conclusions: The rate of neutropenia was higher in a population that received alemtuzumab induction and 6 months of valGCV prophylaxis compared to rates in previous studies that included few patients with alemtuzumab induction (39% vs. 15%) at 6 months. The rates of severe neutropenia and use of G-CSF were also more frequent in the alemtuzumab-induced kidney transplant patients.
Ho, B.: Grant/Research Support, Valganciclovir. Lapin, B.: Grant/Research Support, Valganciclovir. Kamauaa, A.: Grant/Research Support, Valganciclovir. Loveless, M.: Employee, Valganciclovir. Hoop, R.: Employee, Valganciclovir. Ison, M.: Grant/Research Support, Valganciclovir.
To cite this abstract in AMA style:
Nadimpalli L, Ho B, Lapin B, Dalal P, Friedewald J, Kamauaa A, Loveless M, Hoop R, Ladner D, Ison M. 6-Month Universal CMV Prophylaxis and Neutropenia in Kidney Transplant Patients Induced with Alemtuzumab: A Single Center Retrospective Study [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/6-month-universal-cmv-prophylaxis-and-neutropenia-in-kidney-transplant-patients-induced-with-alemtuzumab-a-single-center-retrospective-study/. Accessed July 12, 2025.« Back to 2013 American Transplant Congress
