1-Year Outcomes of a Prospective, Open Label, Randomized, Controlled Trial of Standard vs Extended-Release Tacrolimus as Maintenance Monotherapy in Kidney Transplantation after Alemtuzumab Induction with Rapid Steroid Withdrawal (TAESR Trial)
Imperial College Kidney &
Transplant Centre, Hammersmith Hospital, London, United Kingdom
Imperial Clinical Trials Unit, Imperial College, London, United Kingdom
Department of Medicine, Imperial College, London, United Kingdom
Meeting: 2013 American Transplant Congress
Abstract number: B1098
Background: Extended-release (ER, once daily) tacrolimus (Advagraf) has been shown to provide equivalent results to standard-release (SR, twice daily) tacrolimus in several regimens for maintenance immunosuppression after kidney transplantation, but not in the context of tacrolimus-based maintenance monotherapy.
Methods: We have undertaken a prospective, randomized controlled, investigator-led, single centre, open-label trial comparing ER with SR tacrolimus as maintenance monotherapy after alemtuzumab induction (1x30mg dose) and rapid steroid elimination at 7 days. 50 patients recieved SR twice daily tacrolimus (Prograf) and 52 ER once daily tacrolimus (Advagraf). Randomization was stratified for live vs deceased donors, and the groups were balanced with respect to, age, sex, ethnicity, primary renal diagnosis, and prior sensitisation.
Results: The primary outcome measure was survival with a functioning graft at 1 year, and did not differ significantly between the two groups (SR 96% vs ER 92% p=0.26). Rejection-free survival was SR 84%, ER 86% p=0.48.
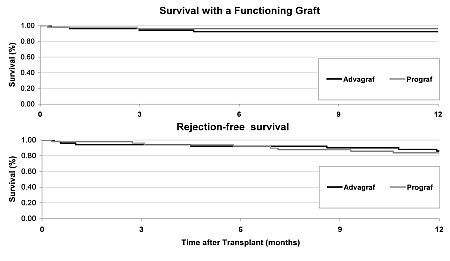
There was no significant difference between the two arms in mean graft function(MDRD-eGFR at 1 year: SR 53.9 ml/min 95%CI 47.0-60.0, ER 54.0 95% CI 46.5-61.5), tacrolimus level (SR 7.73 ng/ml ER 7.39 ng/ml at 1 year) or dispersion of tacrolimus trough levels, or incidence of sub-clinical rejection or degree of interstitial fibrosis in surveillance biopsies taken at 3 months and 1 year post-transplant
Conclusions: Under alemtuzumab induction with rapid steroid elimination, once daily extended-release tacrolimus maintenance monotherapy provides equivalent one-year survival with functioning graft to standard-release twice daily tacrolimus, with a highly simplified once-daily, single-agent regimen.
McLean, A.: Grant/Research Support, Astellas Pharma.
To cite this abstract in AMA style:
McLean A, Chan K, Galliford J, Goodall D, Charif R, Willicombe M, Roufosse C, Cook T, Taube D. 1-Year Outcomes of a Prospective, Open Label, Randomized, Controlled Trial of Standard vs Extended-Release Tacrolimus as Maintenance Monotherapy in Kidney Transplantation after Alemtuzumab Induction with Rapid Steroid Withdrawal (TAESR Trial) [abstract]. Am J Transplant. 2013; 13 (suppl 5). https://atcmeetingabstracts.com/abstract/1-year-outcomes-of-a-prospective-open-label-randomized-controlled-trial-of-standard-vs-extended-release-tacrolimus-as-maintenance-monotherapy-in-kidney-transplantation-after-alemtuzumab-induction-w/. Accessed July 5, 2025.« Back to 2013 American Transplant Congress
