Is C3d-fixing Donor Specific HLA Antibody Detection Really Necessary?
1Service de Néphrologie, Grenoble University Hospital, Grenoble, France
2Laboratoire d'Histocompatibilité, EFS, Grenoble, France
3Service d'Urologie, Grenoble University Hospital, Grenoble, France.
Meeting: 2016 American Transplant Congress
Abstract number: 405
Keywords: HLA antibodies, Kidney transplantation, MHC class II
Session Information
Session Name: Concurrent Session: Kidney AMR: Predicting the Patient at Risk
Session Type: Concurrent Session
Date: Tuesday, June 14, 2016
Session Time: 2:30pm-4:00pm
 Presentation Time: 2:30pm-2:42pm
Presentation Time: 2:30pm-2:42pm
Location: Ballroom B
Introduction: Chronic antibody–mediated rejection is the main cause of late kidney graft loss. The identification of donor-specific antibodies (DSA) in the serum is one of the main criteria for this diagnosis. Single-antigen (SA) Luminex assays provide DSA identification and a semi-quantitative estimate of the amount of antibody by mean fluorescence intensity measurement (MFI). Recent data have shown that patients whose DSA fix C3d have a worse clinical outcome, implying that C3d specific Luminex assays might provide useful prognostic data. In this work, we compared C3dDSA to standard MFI in a cohort of patients having developed de novo class 2 DSA and analyzed graft survival at one year following detection.
Methods: We included kidney graft recipients transplanted between 2005 and 2015 in our center, who developed de novo class 2 DSA. Serum was tested by standard SA Luminex technique and by C3d – fixing antibody detection system (IMMUCOR®) according to the manufacturer's instructions. Clinical data was analyzed regarding graft function and survival at DSA detection and one year later.
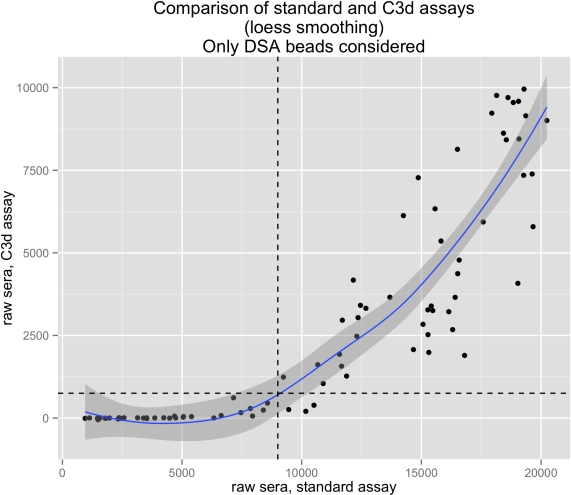
Results: 41/924 patients (4.4%) developed class 2 DSA. 65 serum samples were analyzed. Among them, 43 serum samples were negative for C3dDSA (66%). As shown in figure 1, an MFI threshold of 9000 in SA Luminex assay permitted to discern the negative from the positive C3dDSA. This also holds true when all single bead results are taken into account.
From a clinical standpoint: 23% of patients doubled their creatinine level or lost their graft at one year. C3d positivity was not significantly associated to worse survival.
Conclusion: We show that C3d fixing antibody detection is highly correlated to SA Luminex MFI: in our cohort an MFI threshold of 9000 in SA Luminex predicts C3d positivity. Furthermore we do not find any short-term correlation between C3d positivity and graft survival. We conclude that in this setting assaying for C3d positivity is unnecessary.
CITATION INFORMATION: Malvezzi P, Villemaire M, Bourdin A, Jouve T, Tetaz R, Janbon B, Terrier N, Masson D. Is C3d-fixing Donor Specific HLA Antibody Detection Really Necessary? Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Malvezzi P, Villemaire M, Bourdin A, Jouve T, Tetaz R, Janbon B, Terrier N, Masson D. Is C3d-fixing Donor Specific HLA Antibody Detection Really Necessary? [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/is-c3d-fixing-donor-specific-hla-antibody-detection-really-necessary/. Accessed February 20, 2026.« Back to 2016 American Transplant Congress
