Association of Pre-Transplant Midodrine Use and Complications After Kidney Transplantation.
1Washington University, St. Louis
2Saint Louis University, St. Louis
3Dartmouth Hitchcock Medical Center, Hanover
4Johns Hopkins University, Baltimore.
Meeting: 2016 American Transplant Congress
Abstract number: 156
Keywords: Graft failure, Kidney transplantation, Risk factors
Session Information
Session Name: Concurrent Session: Older and High Risk Kidney Transplant Recipients/Donors
Session Type: Concurrent Session
Date: Sunday, June 12, 2016
Session Time: 4:30pm-6:00pm
 Presentation Time: 5:06pm-5:18pm
Presentation Time: 5:06pm-5:18pm
Location: Ballroom A
Background: Midodrine is increasingly prescribed to decrease the severity and complications of hypotension in dialysis patients. The impacts of midodrine use before kidney transplantation on graft and patient outcomes early after transplantation are not well described.
Methods: We analyzed linked national U.S. transplant registry, pharmacy records and Medicare claims data to follow 16,308 kidney transplant recipients (2005-2008), of whom 308 (1.9%) had filled midodrine prescriptions in the year prior to transplantation. We examined associations of midodrine use with DGF, graft survival and death as reported to the registry, and with clinical complications captured in Medicare claims.
Results: At 3mo, patients who used midodrine before transplant had higher rates of DGF, 32% vs. 19%; hypotension, 14% vs. 4%; acute myocardial infarction (AMI), 4% vs. 2%; cardiac arrest, 2% vs. 0.9%, graft failure, 5% vs. 2%; and death, 4% vs. 1% than the non-users (P<0.05). After multivariate adjustment including recipient, and donor factors, as well as for the propensity of midodrine exposure, pretransplant midodrine use was independently associated with risks of DGF (aOR 1.95; CI 1.49-2.56), death-censored graft failure (aHR 1.94; CI 1.14-3.27), death (aHR 3.55; CI 1.99-6.33), as well as many potentially mediating complications (Figure 1B). Patterns were similar at 12mo. 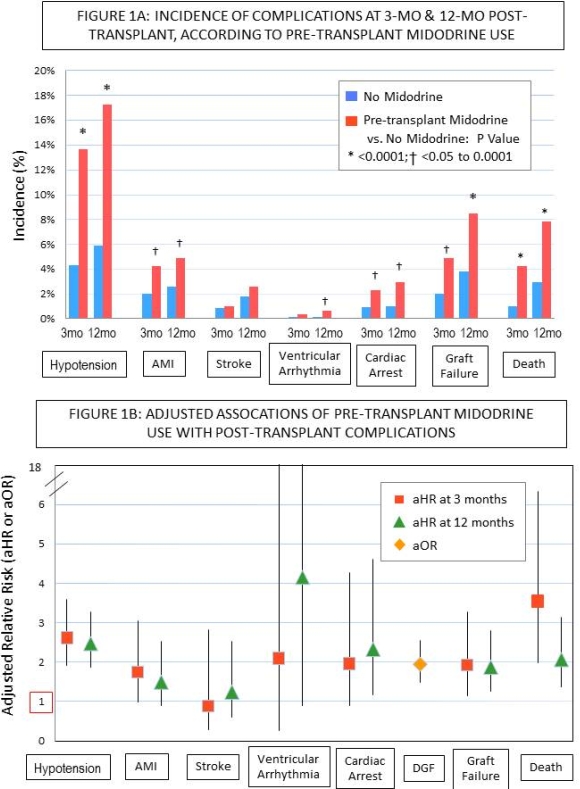
Conclusion: Although associations may in part reflect underlying conditions, the need for midodrine before kidney transplantation is a bio-marker for increased risks of complications including graft failure and death. As the new KAS is expected to increase ESRD duration at the time of allocation for many patients, monitoring recipient comorbidity burden through novel methods including pharmacy claims, and associated impacts on transplant outcomes, are important priorities.
CITATION INFORMATION: Alhamad T, Brennan D, Brifkani Z, Xiao H, Schnitzler M, Dharnidharka V, Axelrod D, Segev D, Lentine K. Association of Pre-Transplant Midodrine Use and Complications After Kidney Transplantation. Am J Transplant. 2016;16 (suppl 3).
To cite this abstract in AMA style:
Alhamad T, Brennan D, Brifkani Z, Xiao H, Schnitzler M, Dharnidharka V, Axelrod D, Segev D, Lentine K. Association of Pre-Transplant Midodrine Use and Complications After Kidney Transplantation. [abstract]. Am J Transplant. 2016; 16 (suppl 3). https://atcmeetingabstracts.com/abstract/association-of-pre-transplant-midodrine-use-and-complications-after-kidney-transplantation/. Accessed February 8, 2026.« Back to 2016 American Transplant Congress
