Outcomes of Eculizumab (ANTI-C5) Therapy for Treatment of Refractory Antiobdy-Mediated Rejection (ABMR) and Thrombotic Microangiopathy (TMA)
1Comprehensive Transplant Center, Cedars-Sinai Medical Center, Los Angeles, CA
2Pathology, Cedars-Sinai Medical Center, Los Angeles, CA.
Meeting: 2015 American Transplant Congress
Abstract number: A95
Keywords: Highly-sensitized, Hyperacute rejection, Kidney transplantation
Session Information
Session Name: Poster Session A: Kidney Antibody Mediated Rejection
Session Type: Poster Session
Date: Saturday, May 2, 2015
Session Time: 5:30pm-7:30pm
 Presentation Time: 5:30pm-7:30pm
Presentation Time: 5:30pm-7:30pm
Location: Exhibit Hall E
Introduction: ABMR results from DSA binding to donor endothelium initiating the classical complement pathway. This results in the formation of terminal MAC (C5b-C9) complex and ultimately cellular lysis and death. Eculizumab (Emab) is a humanized monoclonal antibody that binds to the complement factor C5, inhibiting its cleavage to C5a and C5b, and preventing the formation of the C5-C9 MAC. There are few case reports using Emab for treatment of refractory ABMR and TMA. Here we report efficacy and safety of Emab in treating patients with severe ABMR episodes unresponsive to standard treatment with IVIG + Rituximab +/- PLEX. Patients & Methods: 13 HS patients who presented with severe declines in renal function associated with DSA and/or evidence of ABMR+ on biopsy were included. All initially received standard treatment without response. Patients were then treated with Emab 900-1200 mg weekly for 2-8 doses.Patient & graft survival, renal function (SCr) and DSA levels pre-ABMR, at ABMR and post-ABMR treatment were assessed. Results: 8/13 patients had significant pathologic findings of ABMR, C4d+ (86%) and 5/13 patients had ABMR with TMA. Most patients with ABMR had elevated DSA and/or elevated AT1R antibody at time of treatment. No patient deaths were seen, but 2 graft losses (one severe early ABMR+ and one late ABMR 5 years post-tx secondary to non-adherence). 8/13 patients also had biopsy proven TMA and 1/13 had presumed TMA. 7 TMA patients recovered fully or partially after Emab. This compares with 100% graft failure in TMA+ patients treated with IVIG + rituximab + PLEX. Renal function significantly improved or stabilized in all patients with graft survival post-Emab.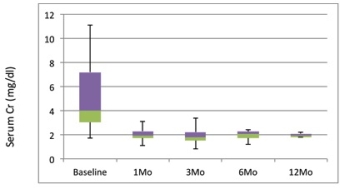 Conclusion: Emab appears to be an important therapeutic option for treatment of severe ABMR associated with rapid decline in renal function unresponsive to standard treatment, especially TMA+. Combining antibody-reduction therapies with Emab will likely yield better outcomes in ABMR.
Conclusion: Emab appears to be an important therapeutic option for treatment of severe ABMR associated with rapid decline in renal function unresponsive to standard treatment, especially TMA+. Combining antibody-reduction therapies with Emab will likely yield better outcomes in ABMR.
To cite this abstract in AMA style:
Wongsaroj P, Choi J, Vo A, Kahwaji J, Peng A, Villicana R, Haas M, Jordan S. Outcomes of Eculizumab (ANTI-C5) Therapy for Treatment of Refractory Antiobdy-Mediated Rejection (ABMR) and Thrombotic Microangiopathy (TMA) [abstract]. Am J Transplant. 2015; 15 (suppl 3). https://atcmeetingabstracts.com/abstract/outcomes-of-eculizumab-anti-c5-therapy-for-treatment-of-refractory-antiobdy-mediated-rejection-abmr-and-thrombotic-microangiopathy-tma/. Accessed March 5, 2026.« Back to 2015 American Transplant Congress
