Safety and Efficacy of Sodium Glucose Cotransporter-2 Inhibitors in Solid Organ Transplant Recipients
University Health, San Antonio, TX
Meeting: 2022 American Transplant Congress
Abstract number: 1663
Keywords: Efficacy, Post-transplant diabetes, Safety
Topic: Clinical Science » Pharmacy » 30 - Non-Organ Specific: Clinical Pharmacy/Transplant Pharmacotherapy
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: To determine the safety and efficacy of sodium glucose cotransporter-2 inhibitors (SGLT2i) in solid organ transplant.
*Methods: This is a retrospective review of adult kidney, liver, and lung transplant recipients at University Health Transplant Institute between 1/1/2010 and 7/31/2021. Patients initiated on an SGLT2i for post-transplant diabetes mellitus (PTDM) for a minimum of 90 days with at least one follow-up appointment were screened for inclusion. Outcomes were evaluated from SGLT2i initiation to nadir 3-12-months post-initiation. The primary outcomes were changes in hemoglobin A1c (HbA1c) and fasting blood glucose (FBG). Secondary outcomes included changes in actual body weight (BW), body mass index (BMI), serum creatinine (SCr), estimated glomerular filtration rate (eGFR), and total insulin daily dose (TDD). Safety outcomes included adverse effects, cardiovascular events, graft loss, and all-cause mortality.
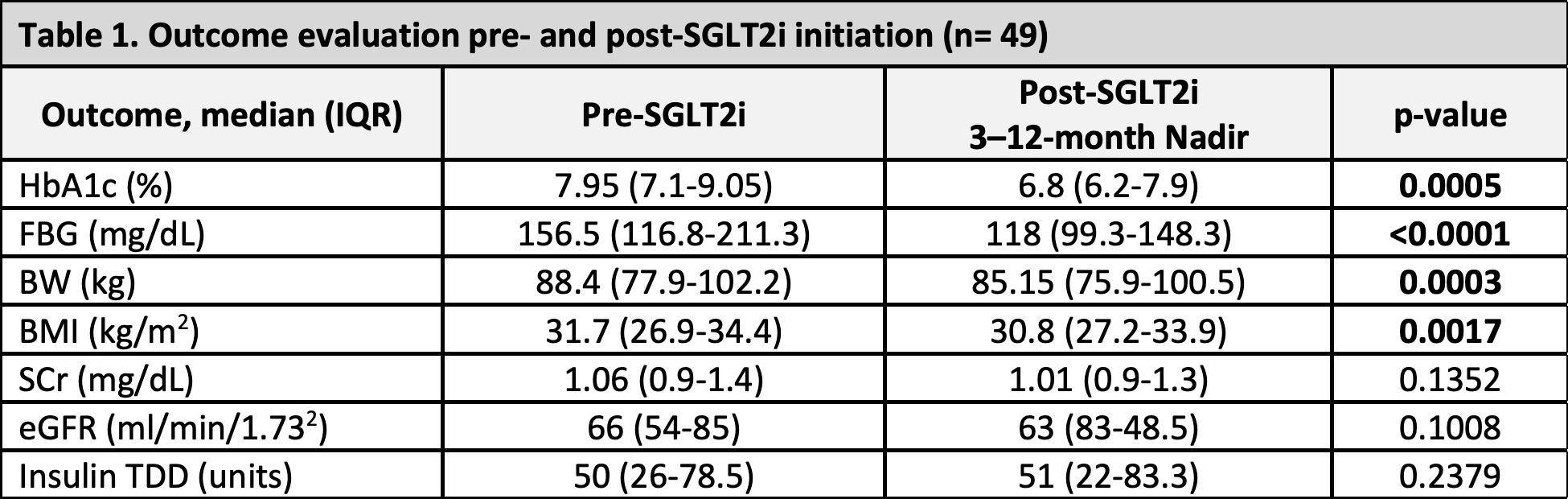
*Results: Forty-nine patients met inclusion criteria, including 26 liver, 18 kidney, 4 lung, and 1 simultaneous liver-kidney recipients. The median time from transplant to SGLT2i initiation was 1216 days [IQR 524-2256] with a median SGLT2i duration of 284 days [IQR 137-520]. Glycemic and weight loss outcomes showed a significant benefit post-SGLT2i (Table 1). No patient experienced myocardial infarctions, graft loss, or mortality at 3-12 months post-SGLT2i. There was only 1 incidence of UTI and stroke. The most common adverse effect included hypotension (n=7) and hypoglycemia (n=10).
*Conclusions: Initiation of SGLT2i post-transplant resulted in significant glycemic and weight loss benefits. These results support the use of this medication class for DM management in this population while maintaining patient safety. Despite concern for UTI incidence with SGLT2i use, especially in immunosuppressed patients, only 1 UTI occurred in this study. Making this non-insulin treatment option available to transplant patients would provide a more beneficial oral DM regimen.
To cite this abstract in AMA style:
Selznick L, Contreras J, Klein K, Long C, Hall R, Bhayana S, Sweiss H. Safety and Efficacy of Sodium Glucose Cotransporter-2 Inhibitors in Solid Organ Transplant Recipients [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/safety-and-efficacy-of-sodium-glucose-cotransporter-2-inhibitors-in-solid-organ-transplant-recipients/. Accessed February 17, 2026.« Back to 2022 American Transplant Congress