Risk for Loss of Hepatitis B Surface Antibody or Hepatitis B Reactivation in Kidney Transplant Recipients Receiving Lymphocyte Depleting Induction
K. Paplaczyk1, C. Kane1, M. Kapugi1, K. Lang1, K. Cunningham1, C. D'Agostino1, J. Boike2
1Pharmacy, Northwestern Memorial Hospital, Chicago, IL, 2Transplant Hepatology, Northwestern Memorial Hospital, Chicago, IL
Meeting: 2022 American Transplant Congress
Abstract number: 1655
Keywords: N/A, Prophylaxis, Simulect, Viral therapy
Topic: Clinical Science » Pharmacy » 30 - Non-Organ Specific: Clinical Pharmacy/Transplant Pharmacotherapy
Session Information
Session Time: 7:00pm-8:00pm
 Presentation Time: 7:00pm-8:00pm
Presentation Time: 7:00pm-8:00pm
Location: Hynes Halls C & D
*Purpose: Although used in over 60% of kidney transplant recipients, the impact of lymphocyte depleting induction on hepatitis B surface antibody (HBsAb) and hepatitis B virus (HBV) reactivation remains unknown. The purpose of this study was to determine if the use of lymphocyte depleting therapy impacts loss of HBsAb or risk of HBV reactivation in kidney transplant recipients and if antiviral therapy is needed
*Methods: This was a retrospective study of adult kidney transplant recipients who received an organ from a hepatitis B core antibody (HBcAb) positive donor. To be included in the study, patients must have received either alemtuzumab or basiliximab for induction immunosuppression. Patients were excluded if they had a non-reactive HBsAb serology on date of transplant, received rituximab or any additional lymphocyte depleting agents within one year post-transplant, or if they were on HBV antiviral therapy. The primary endpoint was HBsAb and hepatitis B surface antigen (HBsAg) serostatus within 3, 6, and 12 months post-transplant. Secondary endpoints included HBV reactivation defined as positive HBsAg or HBV DNA greater than 20 IU/L, biopsy-proven acute rejection (BPAR), graft loss, and death within 1 year post-transplant.
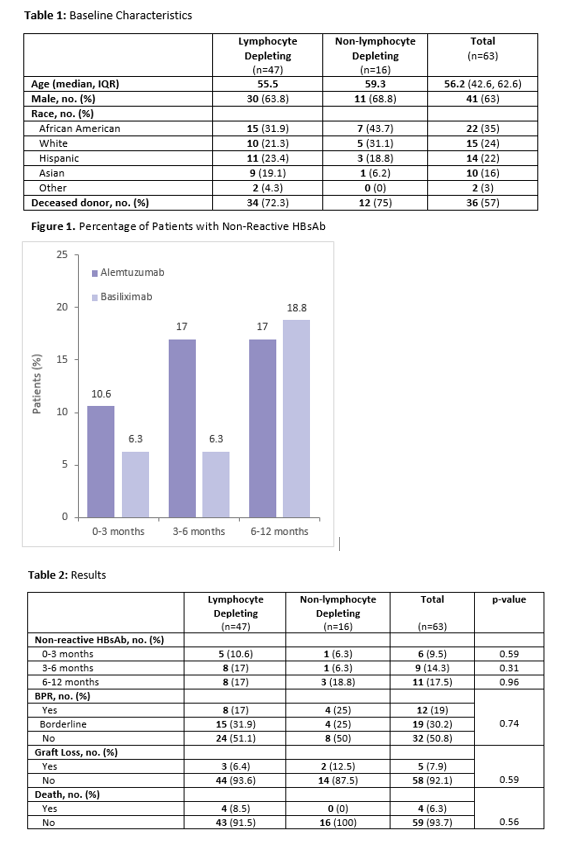
*Results: Sixty-three patients were analyzed. Forty-seven patients (75%) received lymphocyte depleting induction, and 16 patients (25%) received non-lymphocyte depleting induction. Eleven patients lost HBsAb positivity within one year post-transplant. Of those 11 patients, eight received a lymphocyte depleting induction agent (p= 0.9). No patients experienced HBV reactivation. BPAR was observed in 12 patients (p=0.74), five patients experienced graft loss (p=0.59), and four patients died within 1 year post-transplant (p=0.56). No deaths were related to rejection or graft loss.
*Conclusions: Though the rate of loss of HBsAb was numerically higher in the lymphocyte depleting induction cohort, it did not reach statistical significance. No patients experienced HBV reactivation in either cohort and antiviral prophylaxis does not appear to be warranted in this population. Additional, larger studies with longer follow-up should be conducted to determine the significance of these findings.
To cite this abstract in AMA style:
Paplaczyk K, Kane C, Kapugi M, Lang K, Cunningham K, D'Agostino C, Boike J. Risk for Loss of Hepatitis B Surface Antibody or Hepatitis B Reactivation in Kidney Transplant Recipients Receiving Lymphocyte Depleting Induction [abstract]. Am J Transplant. 2022; 22 (suppl 3). https://atcmeetingabstracts.com/abstract/risk-for-loss-of-hepatitis-b-surface-antibody-or-hepatitis-b-reactivation-in-kidney-transplant-recipients-receiving-lymphocyte-depleting-induction/. Accessed February 27, 2026.« Back to 2022 American Transplant Congress